Introduction
Materials and Methods
1. Cell culture
2. Reagents
3. Measurement of the intracellular ROS levels
4. Measurement of cell death
5. Subcellular fractioning
6. Immunoblot analysis
7. Statistical analysis
Results
1. Cell death of the malignant astrocytoma cells by proapoptotic ginsenosides
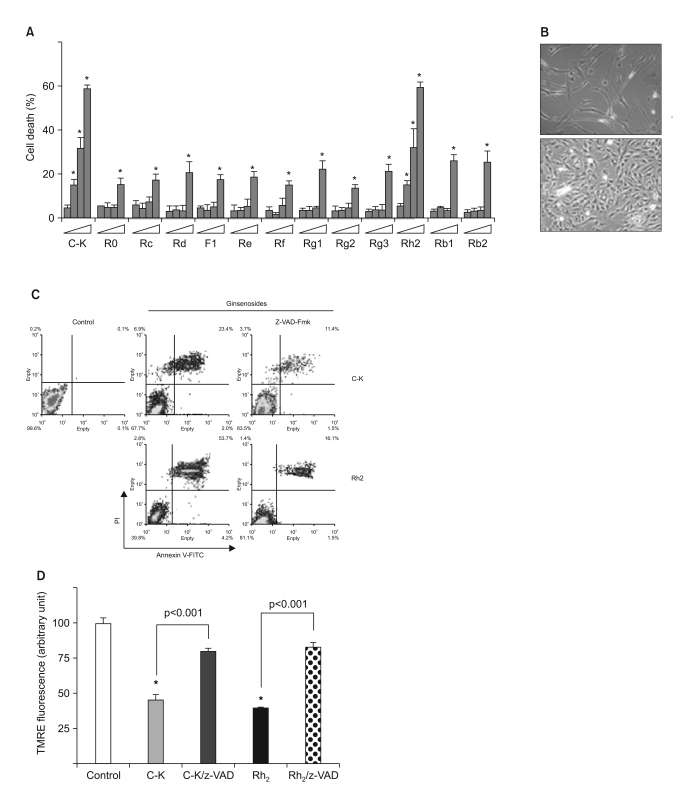 | Fig. 1Caspase-dependent apoptosis by the ginsenosides C-K and Rh2 in human astrocytoma cells. (A) The cells were treated with varying doses (0~50 mg/L) of 13 different ginsenosides, and the cell death was determined by MTT assay. *, significantly different from the control sample without any treatment (p<0.01). (B) Human primary cultured astrocytes and CRT-MG cells were treated with 25 mg/L C-K for 6 h. (C) The cells were incubated in the absence or presence of z-VAD-Fmk (10 mmol/L) for 1 h, and then they were treated with C-K or Rh2 for an additional 24 h, and the cell death was next determined after staining with Annexin-V-FITC and PI by FACS analysis. (D) The cells were incubated in the absence or presence of z-VAD-Fmk (10 mmol/L) for 1 h; they were next treated with C-K or Rh2 for an additional 24 h and then they were stained with TMRE. *, significantly different from the control sample (p<0.001). |
2. The signaling pathways involved in ginsenosides-induced apoptosis
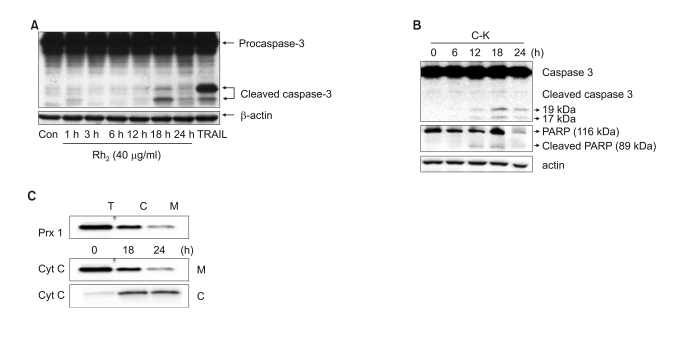 | Fig. 2The involvement of caspase-3 and cytochrome C release in ginsenosides-induced cytotoxicity. (A) CRT-MG cells were incubated with Rh2 for varying time periods, and the cell lysates were subjected to western blot analysis for caspase-3 and b-actin. (B) CRT-MG cells were incubated with C-K for varying time periods, and the cell lysates were subjected to western blot analysis for caspase-3, PARP and b-actin. (C) The total cell lysates (T) were divided into the cytosolic fraction (C) and the mitochondrial fraction (M), and peroxiredoxin-1 was examined to test the separation efficiency (upper panel). The mitochondrial fraction (M) and the cytosolic fraction (C) were tested for their cytochrome-c levels. |
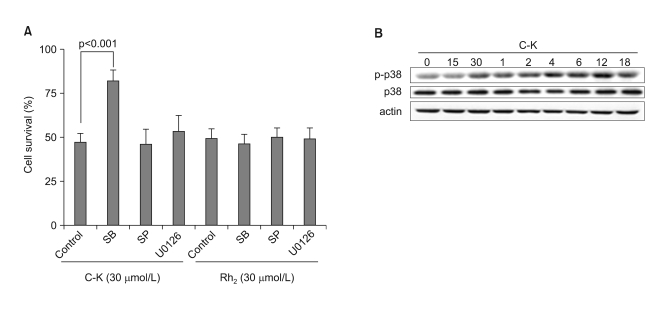 | Fig. 3The involvement of p38MAPK in C-K-mediated cell death. (A) CRT-MG cells were incubated in the absence or presence of various MAPK inhibitors for 1 h, and then they were treated with C-K or Rh2 for an additional 24 h, and cell death was measured by MTT assay. (B) The cells were treated with C-K (25 mg/L) for varying time periods, and the total cell lysates were subjected to western blot analysis for p38 MAPK, phospho-p38 MAPK and β-actin. |
3. The synergistic effect of ginsenosides on Fas-induced cell death
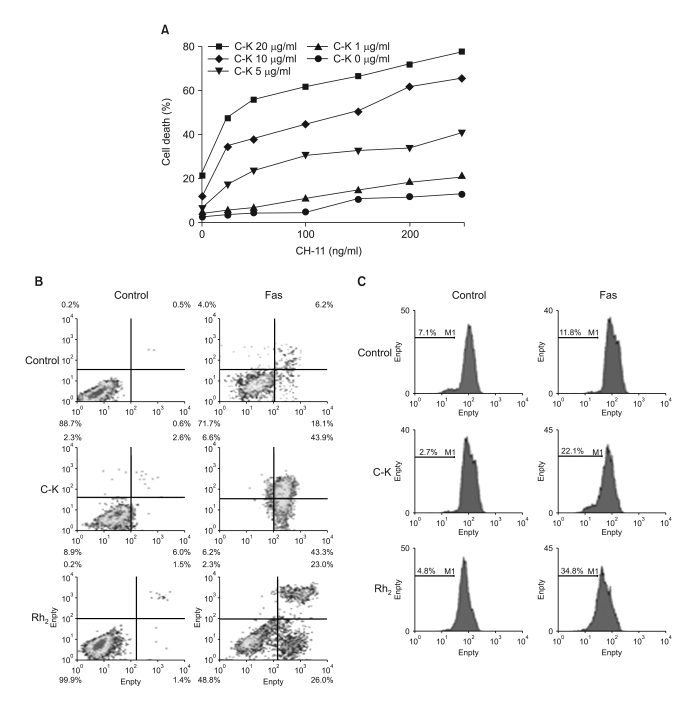 | Fig. 4Ginsenosides augment fas-mediated cell death. (A) The cells were incubated in the absence or presence of varying doses of C-K for 1 h, and then they were treated with CH-11 for an additional 24 h, and the cell death was measured by MTT assay. (B) The cells were incubated in the absence of presence of C-K or Rh2 for 1 h, and then they were treated with CH-11 for an additional 24 h, and the cell death was measured after staining with Annexin-V and PI by FACS analysis. (C) The cells were incubated in the absence or presence of C-K or Rh2 for 1 h, and then they were treated with CH-11 for an additional 24 h, and the Δψm was measured after staining with TMRE. |
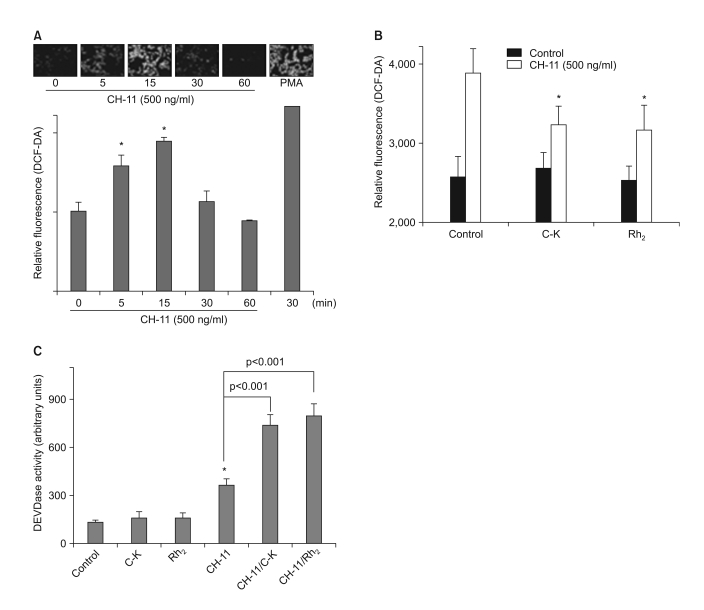 | Fig. 5Ginsenosides Suppress Fas-Induced ROS Generation. (A) The CRT-MG cells were incubated with anti-Fas antibody (CH-11) for varying time periods, and then they were examined for their intracellular ROS levels by staining with DCF-DA. *, significantly different from the control sample (p<0.05). (B) The cells were incubated in the absence or presence of C-K or Rh2 for 1 h, and then they were treated with CH-11 for an additional 30 min, and the intracellular ROS levels were measured. *, significantly different from the control sample treated with CH-11 alone (p<0.01). (C) The cells were incubated in the absence or presence of C-K or Rh2 for 1 h, and then they were treated with CH-11 for an additional 2 h, and the in vitro DEVDase enzymatic activity was measured. *, significantly different from the control sample without any treatment (p<0.01). |
Discussion
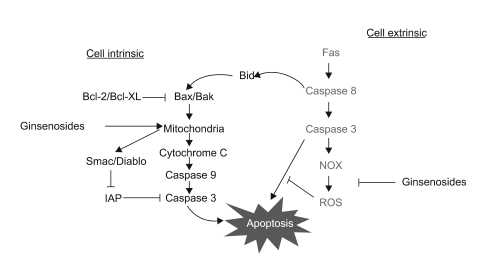 | Fig. 6The proposed model for the anti-tumor activity of ginsenosides and the therapeutic strategies for treating malignant tumors. Ginsenosides induce apoptotic cell death in a mitochondria-dependent manner, while the sensitizing effect of ginsenosides for Fas treatment occurs by ROS inhibition. Since ginsenosides can induce intrinsic cell death and they augment Fas-induced extrinsic cell death, a combined treatment of Fas and ginsenosides might be employed as a useful therapeutic strategy for treating multi-drug resistant malignant astrocytomas. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download