1. Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, et al. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008; 23:351–365. PMID:
18318820.

2. Yasui W, Sentani K, Motoshita J, Nakayama H. Molecular pathobiology of gastric cancer. Scand J Surg. 2006; 95:225–231. PMID:
17249269.

3. Guarino V, Faviana P, Salvatore G, Castellone MD, Cirafici AM, De Falco V, et al. Osteopontin is overexpressed in human papillary thyroid carcinomas and enhances thyroid carcinoma cell invasiveness. J Clin Endocrinol Metab. 2005; 90:5270–5278. PMID:
15998773.

4. Cook AC, Tuck AB, McCarthy S, Turner JG, Irby RB, Bloom GC, et al. Osteopontin induces multiple changes in gene expression that reflect the six 'hallmarks of cancer' in a model of breast cancer progression. Mol Carcinog. 2005; 43:225–236. PMID:
15864800.

5. Irby RB, McCarthy SM, Yeatman TJ. Osteopontin regulates multiple functions contributing to human colon cancer development and progression. Clin Exp Metastasis. 2004; 21:515–523. PMID:
15679049.

6. Tuck AB, Hota C, Wilson SM, Chambers AF. Osteopontin-induced migration of human mammary epithelial cells involves activation of EGF receptor and multiple signal transduction pathways. Oncogene. 2003; 22:1198–1205. PMID:
12606946.

7. Tuck AB, Chambers AF. The role of osteopontin in breast cancer: clinical and experimental studies. J Mammary Gland Biol Neoplasia. 2001; 6:419–429. PMID:
12013531.
8. Murai T, Miyazaki Y, Nishinakamura H, Sugahara KN, Miyauchi T, Sako Y, et al. Engagement of CD44 promotes Rac activation and CD44 cleavage during tumor cell migration. J Biol Chem. 2004; 279:4541–4550. PMID:
14623895.

9. Philip S, Kundu GC. Osteopontin induces nuclear factor κB-mediated promatrix metalloproteinase-2 activation through I
κB
α/IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways. J Biol Chem. 2003; 278:14487–14497. PMID:
12473670.
10. Philip S, Bulbule A, Kundu GC. Osteopontin stimulates tumor growth and activation of promatrix metalloproteinases-2 through nuclear factor-kappa B-mediated induction of membrane type 1 matrix metalloproteinase in murine melanoma cells. J Biol Chem. 2001; 276:44926–44935. PMID:
11564733.
11. Renault MA, Jalvy S, Potier M, Belloc I, Genot E, Dekker LV, et al. UTP induces osteopontin expression through a coordinate action of NFkB, activator protein-1, and upstream stimulatory factor in arterial smooth muscle cells. J Biol Chem. 2005; 280:2708–2713. PMID:
15557322.
12. Samant RS, Clark DW, Fillmore RA, Cicek M, Metge BJ, Chandramouli KH, et al. Breast cancer metastasis suppressor 1 (BRMS1) inhibits osteopontin transcription by abrogating NFkB activation. Mol Cancer. 2007; 6:6–15. PMID:
17227585.

13. Kim JY, Lim S, Park K. Cyclooxygenase-2 and c-erbB-2 expression in gastric carcinoma assessed using tissue microarrays. Appl Immunohistochem Mol Morphol. 2004; 12:67–70. PMID:
15163022.
14. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2
-ΔΔCT method. Methods. 2001; 25:402–408. PMID:
11846609.
15. Weber GF. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochem Biophys Acta. 2001; 1552:61–85. PMID:
11825687.

16. Eschrich S, Yang I, Bloom G, Kwong KY, Boulware D, Cantor A, et al. Molecular staging for survival prediction of gastric cancer patients. J Clin Oncol. 2005; 23:3526–3535. PMID:
15908663.
17. Wu CY, Wu MS, Chiang EP, Wu CC, Chen YJ, Chen CJ, et al. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007; 56:782–789. PMID:
17148500.

18. Coppola D, Szabo M, Boulware D, Muraca P, Alsarraj M, Chambers AF, et al. Correlation of osteopontin protein expression and pathological stage across a side variety of tumor histologies. Clin Cancer Res. 2004; 10:184–190. PMID:
14734468.
19. Selkirk SM, Morrow J, Barone TA, Hoffer A, Lock J, DeChant A, et al. Elevation of osteopontin levels in brain tumor cells reduces burden and promotes survival through the inhibition of cell dispersal. J Neurooncol. 2008; 86:285–296. PMID:
17928956.

20. Matsuzaki H, Shima K, Muramatsu T, Ro Y, Hashimoto S, Shibahara T, et al. Osteopontin as biomarker in early invasion by squamous cell carcinoma in tongue. J Oral Pathol Med. 2007; 36:30–34. PMID:
17181739.

21. Lee JL, Wang MJ, Sudhir PR, Chen GD, Chi CW, Chen JY. Osteopontin promotes integrin activation through outside-in and inside-out mechanism:OPN-CD44v interaction enhances survival in gastrointestinal cancer cells. Cancer Res. 2007; 67:2089–2097. PMID:
17332338.
22. Wai PY, Mi Z, Guo H, Sarraf-Yazdi S, Gao C, Wei J, et al. Osteopontin silencing by small interfering RNA suppresses in vitro and in vivo CT26 murine colon adenocarcinoma metastasis. Carcinogenesis. 2005; 26:741–751. PMID:
15661802.

23. Bourguignon LY, Xia W, Wong G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates beta-catenin signaling and NFkB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J Biol Chem. 2009; 284:2657–2671. PMID:
19047049.
24. Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002; 16:3074–3086. PMID:
12464636.

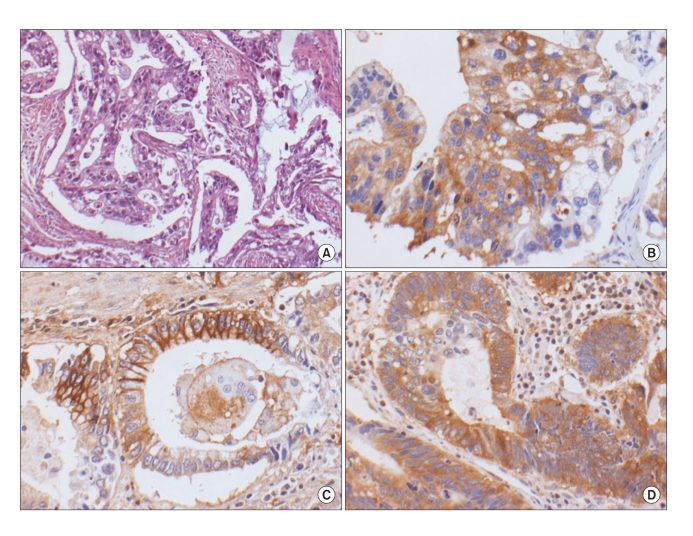




 PDF
PDF Citation
Citation Print
Print


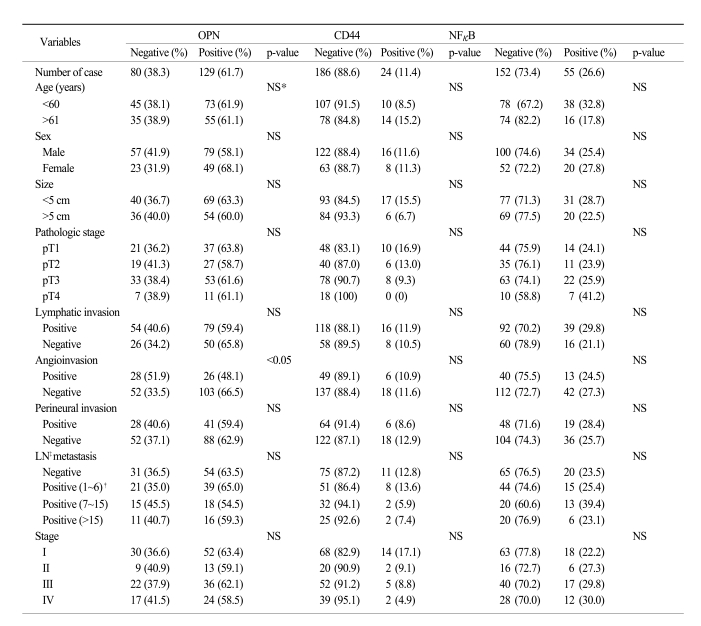
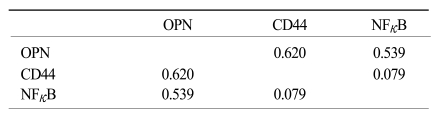
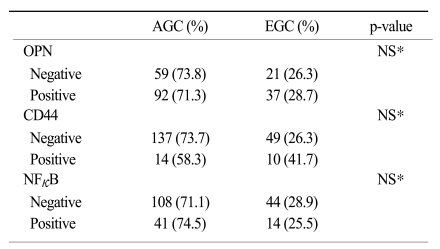
 XML Download
XML Download