Abstract
Purpose
Although the vascular endothelial growth factor (VEGF) superfamily has been identified to critically influence tumor-related angiogenesis, the prognostic significance of a VEGF expression in gastric cancer is still controversial. Accordingly, the present study analyzed the VEGF-A and VEGF-C expressions and their impact on the prognosis of patients with gastric cancer.
Materials and Methods
Three hundred seventy-five consecutive patients who underwent surgical resection for gastric adenocarcinoma with a curative intent were enrolled in the present study. Immunohistochemical staining for VEGF-A and VEGF-C was performed using the formalin fixed, paraffin embedded tumor tissues.
Results
Positive VEGF-A and VEGF-C expressions were observed in 337 (90.1%) and 278 (74.9%) cases, respectively. The survival analysis showed that the expression of VEGF-A and VEGF-C had no effect on the OS and DFS. On the multivariate analysis that included age, gender and the TNM stage, no significant association between the grade of the VEGF-A or VEGF-C expression and survival was observed.
Go to : 
Despite its decreasing incidence, gastric cancer is still the second leading cause of cancer-related death worldwide, and particularly in Asian countries (1). The prognosis of patient with gastric cancer has been shown to be influenced by several established surgical-pathological features, such as the pathological stage, the location of the tumor and the histological type and grade of the tumor (2).
Angiogenesis is the formation of new blood vessels from endothelial precursors, and this is a prerequisite for the growth and progression of solid malignancies. The vascular endothelial growth factor (VEGF) superfamily of endothelial growth factors has been identified to critically influence tumor-related angiogenesis (3,4). VEGF-A has the most potent mitotic activity specific to vascular endothelial cells among the many angiogenic factors (5), and VEGF-C, which is a ligand for VEGR receptor (VEGFR)-2 and VEGFR-3, is supposed to take part in both lymphangiogenesis and angiogenesis (6,7). Clinical studies have demonstrated that an increased expression of VEGF or its family is associated with the grade of angiogenesis and the prognosis for various human cancers (8-11). In particular, for gastric cancer, the expression of VEGF-A or VEGF-C has been reported to be correlated with a poor prognosis. For example, Yonemura et al. demonstrated that high levels of a VEGF-C expression were associated with a poorer prognosis and decreased survival for 117 patients with gastric cancer (12). Further significant differences in survival that are associated with the VEGF-C status have been reported by Takahashi et al. in a group of 65 cancer patients (13). However, most of the previous studies included a relatively small sample size to draw a definitive conclusion, and several studies reported that the expression of VEGF-A or VEGF-C was not associated with the prognosis of patients with gastric cancer (14,15). Accordingly, the present study analyzed the VEGF-A and VEGF-C expressions and their impact on the prognosis in a large population of patients with surgically resected gastric cancer.
Go to : 
All the tissues investigated in this study were obtained from consecutive Korean patients (n=375) who had undergone a surgical gastrectomy between January, 2000 and December, 2001 at Kyungpook National University Hospital (Daegu, Korea). The diagnosis and staging of gastric carcinoma were assessed according to the WHO classifications (16) and the TMN classifications set out by the American Joint Committee on Cancer (AJCC) (17). We retrospectively obtained the information concerning the date of the diagnosis, the pathologic staging, relapse and death.
Formalin fixed, paraffin embedded serial sections (4 µm) mounted on 3-aminopropyltriethoxysilane-coated slides (Matsunami Glass Ind. Ltd. Japan) were deparaffinized in xylene and a series of graded alcohol solutions. Rabbit polyclonal anti-VEGF-A (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and anti-VEGF-C (Zymed Laboratories, South California, CA) antibodies were used at concentrations of 1.6 µg/mL and 2.0 µg/mL, respectively. The immunohistochemistry was performed with using a catalyzed signal-amplification system (DAKO, Ely, UK) according to the manufacturer's protocol. The sections were treated with 0.3% hydrogen peroxide (H2O2) in water for 10 minutes to quench any endogenous peroxidase activity within the tissue, and the nonspecific binding sites were blocked with 20% heat-inactivated nonserum protein for 10 minutes at room temperature. Next, the sections were incubated for 15 minutes in the presence of the primary antibody, and then the slides were washed in phosphate buffered saline (PBS) containing 0.1% Tween 20 (PBS/Tween) for 15 minutes while changing the solution 3 times before the application of the secondary biotinylated antibody. The slides were incubated with the secondary antibody for 15 minutes at room temperature before being washed for 15 minutes in PBS/Tween that was changed 3 times. The sections were then incubated for 15 minutes with an avidin-biotinylated-horseradish peroxidase complex, and the reaction visualized using 0.02% 3,3'-diaminobenzidine tetrahydrochloride as a chromogen in a Tris-HCl buffer, pH 7.6, containing 0.03% H2O2. Hematoxylin was used to counterstain the nuclei. The staining results for VEGF-A and VEGF-C were classified by estimating the percentage of epithelial cells that showed specific immunoreactivity: grade 0 (0% to 10% positive cells), grade 1 (10% to 50% positive cells), and grade 2 (>50% positive cells). Two pathologists (H.B. and G.Y.), who were kept "blind" to the patients' characteristics, examined all the slides.
The correlation between the level of the VEGF-A or VEGF-C protein expression and the clinicopathological features was analyzed using Chi-square tests and logistic regression analysis. Overall survival (OS) was measured from the day of surgery until the date of death or the last follow-up. Disease-free survival (DFS) was calculated from the date of surgery until tumor recurrence or death from any cause. The survival estimates were calculated using the Kaplan-Meier method and then they were compared using log-rank statistics. The Cox proportional hazard regression model was used for the multivariate survival analyses, and the analyses were always adjusted for age (<60 versus ( 60 years), gender (male versus female) and the stage (0 to IV). The multivariate analyses were performed on 371 patients for whom the immunohistochemical staining for both VEGF-A and VEGF-C was available. A cut-off p-value of 0.05 was adopted for all the statistical analyses. The statistical data was obtained using a SPSS software package (SPSS 11.5 Inc. Chicago, IL).
Go to : 
The median age of the patients was 60 years (range: 25~83 years), and 247 (65.9%) patients were male. Curative resections were performed in 355 (94.7%) patients, while the others received a palliative gastrectomy. The pathologic stages after the surgical resection were as follows: stage IA (n=92, 24.5%), stage IB (n=84, 22.4%), stage II (n=98, 26.1%), stage IIIA (n=42, 11.2%), stage IIIB (n=23, 6.1%) and stage IV (n=36, 9.6%). Among the 199 patients with stage II to IV disease, 139 (69.8%) patients received adjuvant chemotherapy with 4 cycles of 5-fluorouracil+epirubicin (n=64) or nimustine (n=11) followed by oral 5-fluorouracil for 1 year, or just oral 5-fluorouracil for 1 year (n=64) (Table 1). At the time of the last analysis (December 2008), 140 patients had experienced disease relapse and 111 patients had died as a result of gastric cancer. However, the death of 12 patients was not related to gastric cancer. The 5-year OS and DFS for all the patients were 69.1±2.4% and 66.3±2.5%, respectively.
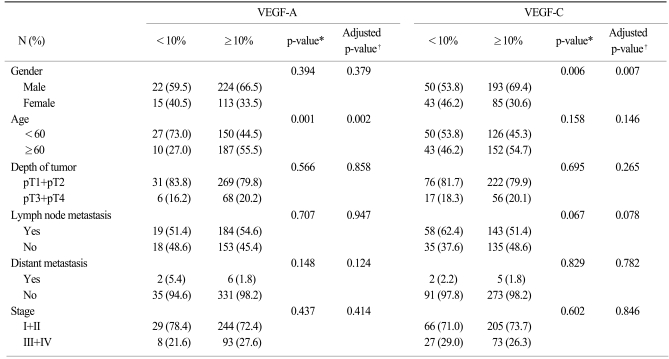
Immunohistochemical staining for the VEGF-A and VEGF-C expressions was successfully performed in 374 (99.7%) and 371 (98.9%) cases, respectively. Three hundred thirty-seven cases (90.1%) were classified as a grade 1 or 2 VEGF-A expression, and 278 cases (74.9%) were classified as a grade 1 or 2 VEGF-C expression (Table 1). No correlation was observed between the VEGF-A and VEGF-C expressions.
The relationship between the level of the VEGF-A or VEGF-C expression and the clinicopathological features is shown in Table 2. The positive expression of VEGF-A was more common in older patients (p=0.002), whereas the VEGF-C expression was more common in male gender (p=0.007). However, no significant correlation was observed between the VEGF-A or VEGF-C expression and the other pathological features on multivariate analysis.
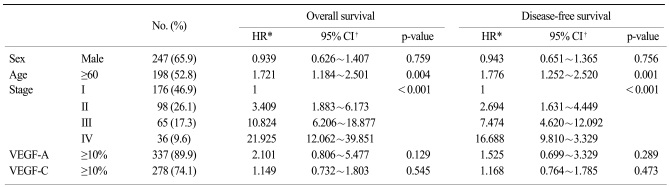
Survival analysis showed that the expressions of VEGF-A and VEGF-C had no effect on OS and DFS (Fig. 1). On the multivariate analysis that included age, gender and the TNM stage, no significant association between the grade of the VEGF-A or VEGF-C expression and survival was observed (Table 3). The results were similar when dichotomized to grade 0 and grade 1 versus a grade 2 expression of VEGF-A or VEGF-C (data not shown). The pathologic stage and an older age were the independent prognostic factors of survival for patients with resected gastric cancer.
Go to : 
We have investigated the prognostic impact of the VEGF-A and VEGF-C expressions in 375 patients with surgically resected gastric adenocarcinoma. The current study demonstrated that the expression of VEGF-A or VEGF-C was not associated with survival for these patients.
Since VEGF-A and VEGF-C are known to play major roles in the growth and metastasis of malignant tumors, many studies have focused on the clinical significance of tumors' VEGF-A or VEGF-C expression or the serum level of these factors for predicting lymph node metastasis and the prognosis of solid tumors, including gastric cancer. The tumor VEGF expression has been reported to be a significant marker for tumor recurrence or reduced survival and this was independent of the conventional clinicopathological factors for gastric cancer (14,18,19). Furthermore, lymphatic invasion and the lymph node status were positively correlated with the tissue expression of VEGF-C in gastric cancer (12-14,20,21). In addition, a positive VEGF-C tissue expression in early gastric cancer was significantly associated with lymphatic invasion, and this could potentially help to predict those individuals who would benefit from more or less extensive surgical resections (20). However, the clinical impact of the association between the VEGF-A or VEGF-C expression and the prognosis is not fully understood. For example, Tanigawa et al. (15) reported that the overall survival rates were not significantly different according to the level of the VEGF-A expression in 163 gastric carcinomas, although the level of the VEGFA expression was correlated with tumor neovascularization. Moreover, no significant trends towards reduced survival for patients with VEGF-C expressing gastric cancers have been found (14). In the present study, the tissue expression of VEGF-A or VEGF-C was not found to be an independent prognostic factor in a quite large population of patients with resected gastric cancer, although the patient survival was significantly different according to the VEGF-A expression on univariate analysis. One possible explanation for these results is that although VEGF-A and VEGF-C play an important role in the angiogenesis and lymphangiogenesis of tumors, a higher tissue VEGF-A or VEGF-C expression may be associated with advanced disease, and so this did not affect survival on the multivariate analysis as adjusted for the pathologic stage. Furthermore, the development of consistent experimental methodology is essential to allow comparison between different studies. This must include the use of antibodies of defined specificity, consistent immunohistochemical protocols with appropriate use of controls and a widespread consensus for the scoring techniques. Accordingly, further studies that will use combinations of new angiogenic or lymphatic markers and functional assays are needed to clarify the relationship between the tissue VEGF-A or VEGF-C expression and survival. Since almost 50% of the patients in our study were stage I and the median OS was not reached for the entire population, it might be too early to analyze this data and draw a conclusion, and so long term follow up study will be needed.
Go to : 
The tissue expression of VEGF-A or VEGF-C alone is not an independent prognostic marker for Korean patients with surgically resected gastric adenocarcinoma. Accordingly, further understanding of the function and actions of VEGF-A and VEGF-C is warranted to optimize the therapeutic strategies for treating gastric cancer and to avoid unwanted side effects.
Go to : 
References
1. Anderson C, Nijagal A, Kim J. Molecular markers for gastric adenocarcinoma: an update. Mol Diagn Ther. 2006; 10:345–352. PMID: 17154651.
2. Miyahara R, Niwa Y, Matsuura T, Maeda O, Ando T, Ohmiya N, et al. Prevalence and prognosis of gastric cancer detected by screening in a large Japanese population: data from a single institute over 30 years. J Gastroenterol Hepatol. 2007; 22:1435–1442. PMID: 17573829.
3. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000; 407:242–248. PMID: 11001067.

4. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000; 407:249–257. PMID: 11001068.

5. Takashima S, Matsushita T, Takayama F, Kadoya M, Fujimori M, Kobayashi T. Prognostic significance of magnetic resonance findings in advanced papillary thyroid cancer. Thyroid. 2001; 11:1153–1159. PMID: 12186503.

6. Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996; 15:1751. PMID: 8612600.

7. Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, et al. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci U S A. 1998; 95:14389–14394. PMID: 9826710.

8. Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol. 2001; 2:667–673. PMID: 11902537.

9. Masuya D, Huang C, Liu D, Kameyama K, Hayashi E, Yamauchi A, et al. The intratumoral expression of vascular endothelial growth factor and interleukin-8 associated with angiogenesis in nonsmall cell lung carcinoma patients. Cancer. 2001; 92:2628–2638. PMID: 11745198.

10. Fontanini G, Faviana P, Lucchi M, Boldrini L, Mussi A, Camacci T, et al. A high vascular count and overexpression of vascular endothelial growth factor are associated with unfavourable prognosis in operated small cell lung carcinoma. Br J Cancer. 2002; 86:558–563. PMID: 11870537.

11. Nishida N, Yano H, Komai K, Nishida T, Kamura T, Kojiro M. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 2 are related closely to the prognosis of patients with ovarian carcinoma. Cancer. 2004; 101:1364–1374. PMID: 15368324.

12. Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res. 1999; 5:1823–1829. PMID: 10430087.
13. Takahashi A, Kono K, Itakura J, Amemiya H, Feng Tang R, Iizuka H, et al. Correlation of vascular endothelial growth factor-C expression with tumor-infiltrating dendritic cells in gastric cancer. Oncology. 2002; 62:121–127. PMID: 11914597.

14. Ichikura T, Tomimatsu S, Ohkura E, Mochizuki H. Prognostic significance of the expression of vascular endothelial growth factor (VEGF) and VEGF-C in gastric carcinoma. J Surg Oncol. 2001; 78:132–137. PMID: 11579392.

15. Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T. Correlation between expression of vascular endothelial growth factor and tumor vascularity, and patient outcome in human gastric carcinoma. J Clin Oncol. 1997; 15:826–832. PMID: 9053510.

16. Hamilton SR, Aaltonen LA. WHO classification. Tumours of the digestive system: pathology & genetics. 2000. 2nd ed. Lyon France: IARC Press.
17. Greene FL, Page DL, Fleming ID. The AJCC cancer staging manual. 2002. 6th ed. New York: Springer-Verlag.
18. Maeda K, Kang SM, Onoda N, Ogawa M, Kato Y, Sawada T, et al. Vascular endothelial growth factor expression in preoperative biopsy specimens correlates with disease recurrence in patients with early gastric carcinoma. Cancer. 1999; 86:566–571. PMID: 10440683.

19. Saito H, Tsujitani S, Kondo A, Ikeguchi M, Maeta M, Kaibara N. Expression of vascular endothelial growth factor correlates with hematogenous recurrence in gastric carcinoma. Surgery. 1999; 125:195–201. PMID: 10026754.

20. Kinoshita J, Kitamura K, Kabashima A, Saeki H, Tanaka S, Sugimachi K. Clinical significance of vascular endothelial growth factor-C (VEGF-C) in breast cancer. Breast Cancer Res Treat. 2001; 66:159–164. PMID: 11437102.

21. Amioka T, Kitadai Y, Tanaka S, Haruma K, Yoshihara M, Yasui W, et al. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur J Cancer. 2002; 38:1413–1419. PMID: 12091074.

Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download