Abstract
Plasmablastic lymphoma (PBL) of the oral cavity is an acquired immunodeficiency syndrome-related lymphoma. The immunophenotype of this disease is associated with poor expression of B-cell markers but a positive reactivity for plasma cell markers. PBL is highly aggressive and responds poorly to treatment. Although originally described in the oral cavity, this disease can occur in other body niches. Here, we describe a very rare case of PBL in the anal canal of a 40-year-old woman with human immunodeficiency virus infection. The malignant cells were positive for Epstein-Barr virus and human herpes virus 8.
Human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) are associated with an increased risk for the development of lymphoproliferative disorders. On the basis of morphologic, immunologic and clinical features, one such disorder designated plasmablastic lymphoma (PBL) was initially proposed to be a distinct variant of diffuse large B-cell lymphoma (1). The consistent presence of plasma cell markers and absence of B-cell markers are the main pathologic features of PBL. The primary presentation site is the oral cavity. Although extra-oral PBLs have been reported, these are considered to be very rare. Here we report a case of anal PBL and provide with a comprehensive review of the relevant literature.
A 47-year-old premenopausal woman was diagnosed with HIV infection in 1996. A diagnosis of AIDS was made in 2000 after the patient had developed tuberculosis. The patient was also found to have a decreased CD4 count. Although the patient received intermittent highly active antiretroviral therapy (HAART), personal compliance was poor, and the medication was frequently not taken.
In 2008, the patient developed anal pain and bleeding that persisted for approximately 1 year. At presentation, the HIV viral load was 143724/mL and the absolute CD4 count was l<200/mm3. During the previous year, the patient had sought medical attention several times at a local hospital, and each time had been diagnosed with an anal fistula. On each occasion, the provision of pain medication and supportive care brought temporary relief. However, perianal discharge and pain had continued for a month prior to admission to our hospital. With the exception of AIDS, the patient she had no prior history of diseases such as diabetes, hypertension, or hepatitis.
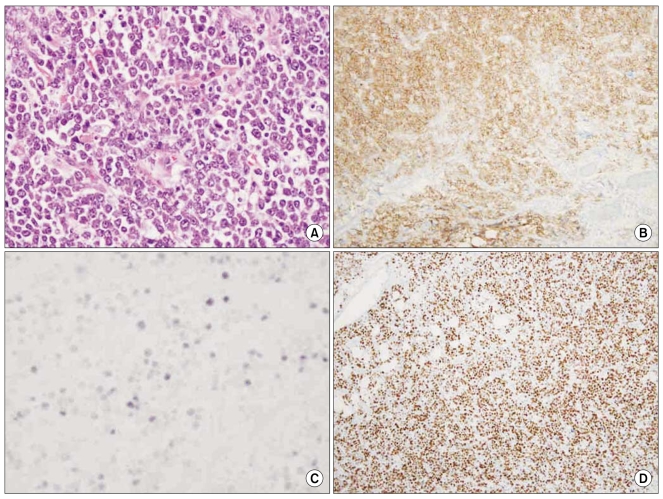
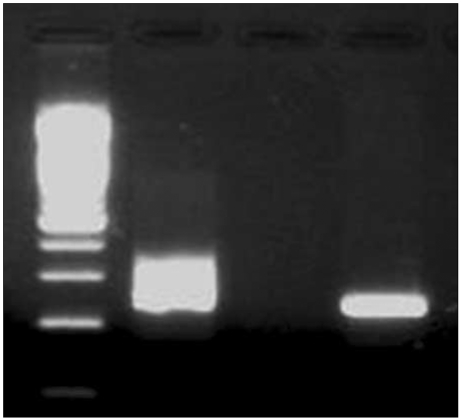
Perianal inspection revealed an anal fistula. Digital rectal examination showed no induration, but a 2 cm palpable mass together with a fibrous tract that extended was into the anal canal was detected. Results of laboratory analyses conducted on admission were: white cell count 4,800/µL; hemoglobin 11.7 g/dL; platelet count 159,000/µL; lactate dehydrogenase 162 U/L (reference range 106~211); calcium 8.5 mg/dL; uric acid 3.3 mg/dL; blood urea nitrogen 14.0 mg/dL; creatinine 0.7 mg/dL; aspartate aminotransferase 21 IU/L; and alanine transminase 22 IU/L. Computed tomography (CT) of the abdomen and pelvis showed an approximately 3 cm anal fistula with a 2 cm anal abscess (Fig. 1). There was no enlarged lymph node in the abdomen, including the perianal area. An anal fistulotomy was performed and a specimen of the anal fistula revealed hypercellularity and the presence of numerous large nuclei. Histopathological examination of cells revealed eccentric nuclei and plasmacytoid features such as abundant basophilic cytoplasm. Immunohistochemical studies were performed using fixed, paraffin-embedded tissue sections. The lymphoid infiltrate was positive for the plasma cell marker CD138, while tumor cells were negative for the B-cell marker CD20 and the T-cell markers CD3 and CD5. Use of polymerase chain reaction (PCR) to amplify the immunoglobulin heavy chain (IgH) gene indicated a monoclonal pattern. The proliferation index (Ki-67) was high, being approximately 95%. In situ hybridization was positive for Epstein-Barr virus (EBV) small nuclear RNAs (EBER) (Fig. 2). The presence of human herpes virus 8 (HHV-8) was confirmed by PCR-based HHV-8 sequence analysis (Fig. 3). On the basis of these findings, a definitive diagnosis of PBL was made.
The patient received three cycles of chemotherapy with a combination of cyclophosphamide, doxorubicin, vincristine (formerly Oncovin®), and prednisolone (CHOP) and HAART after the diagnosis of PBL. Treatment has continued to the present.
Non-Hodgkin lymphoma is the second most common malignancy in HIV-positive patients. PBL was initially described as a rare variant of diffuse large B-cell lymphoma, characteristically diagnosed in patients infected with HIV (1). Although PBL most frequently presents in the oral cavity, with local invasion and rapid dissemination to extra-oral sites, it has been reported in other sites such as the stomach (2), cervical lymph nodes (3), lungs (4), orbit (5), and paranasal sinuses (6). Extra-oral PBLs are rare, but are a concern since they appear to have a similar invasive and rapidly disseminative capability as PBL of the oral cavity. More recently, PBL has been reported in immunocompromised patients who are HIV-negative (7,8). The histological profile of PBL consists of differentiated plasma cells with either absent or weak expression of leukocyte common antigen and B-cell markers such as CD20 and CD45, but expression of plasma cell markers such as CD38 and CD138 (9,10). Characteristic marker expression and immature cell morphology account for the designation of these lymphomas as plasmablasts (11). PBL is likely associated with up to 60% of cases of latent EBV since PBL is often associated with an immuno-deficiency such as HIV infection. EBV might play an important role in the pathogenesis of PBL. Support for this view comes from a study in which an in situ hybridization technique for EBV and PCR for HHV8 revealed EBV in nine of 15 PBL patients, but in none of the three patients positive for HHV8 (1). Another study also failed to confirm the association between HHV8 and PBL (12). However, in a contradictory report, all four cases of oral PBL were PCR-Positive for HHV8 (13). Presently, we detected HHV-8 in lymphoma tissue by PCR. However, we could not determine whether PBL was a direct result of HHV-8 infection, or was present merely by coincidence. Further studies will be needed to ascertain whether HHV8 is influential in the etiology of PBL.
The prognosis for those with PBL is dim; most patients die within the first year following diagnosis (1,10). CHOP combination chemotherapy has been the most commonly used therapeutic regimen. However, given the high proliferation index of PBL and its aggressive behavior, more intensive combination regimens such as CODOX-M/IVAC can be used (14). Prospective studies are warranted to further investigate the pathogenetic mechanisms, prognostic factors, and management of this rare and aggressive HIV-associated lymphoma.
References
1. Delecluse HJ, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, Schneider U, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997; 89:1413–1420. PMID: 9028965.

2. Pruneri G, Graziadei G, Ermellino L, Baldini L, Neri A, Buffa R. Plasmablastic lymphoma of the stomach. A case report. Haematologica. 1998; 83:87–89. PMID: 9542326.
3. Lin F, Zhang K, Quiery AT Jr, Prichard J, Schuerch C. Plasmablastic lymphoma of the cervical lymph nodes in a human immunodeficiency virus-negative patient: a case report and review of the literature. Arch Pathol Lab Med. 2004; 128:581–584. PMID: 15086296.

4. Lin Y, Rodrigues GD, Turner JF, Vasef MA. Plasmablastic lymphoma of the lung: report of a unique case and review of the literature. Arch Pathol Lab Med. 2001; 125:282–285. PMID: 11175653.
5. Valenzuela AA, Walker NJ, Sullivan TJ. Plasmablastic lymphoma in the orbit: case report. Orbit. 2008; 27:227–229. PMID: 18569836.

6. Schichman SA, McClure R, Schaefer RF, Mehta P. HIV and plasmablastic lymphoma manifesting in sinus, testicles, and bones: a further expansion of the disease spectrum. Am J Hematol. 2004; 77:291–295. PMID: 15495247.

7. Teruya-Feldstein J, Chiao E, Filippa DA, Lin O, Comenzo R, Coleman M, et al. CD20-negative large-cell lymphoma with plasmablastic features: a clinically heterogenous spectrum in both HIV-positive and -negative patients. Ann Oncol. 2004; 15:1673–1679. PMID: 15520070.

8. Lin O, Gerhard R, Zerbini MC, Teruya-Feldstein J. Cytologic features of plasmablastic lymphoma. Cancer. 2005; 105:139–144. PMID: 15803491.

9. Campo E, Chott A, Kinney MC, Leoncini L, Meijer CJ, Papadimitriou CS, et al. Update on extranodal lymphomas. Conclusions of the workshop held by the EAHP and the SH in Thessaloniki, Greece. Histopathology. 2006; 48:481–504. PMID: 16623775.

10. Dong HY, Scadden DT, de Leval L, Tang Z, Isaacson PG, Harris NL. Plasmablastic lymphoma in HIV-positive patients: an aggressive Epstein-Barr virus-associated extramedullary plasmacytic neoplasm. Am J Surg Pathol. 2005; 29:1633–1641. PMID: 16327436.
11. Vega F, Chang CC, Medeiros LJ, Udden MM, Cho-Vega JH, Lau CC, et al. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Mod Pathol. 2005; 18:806–815. PMID: 15578069.

12. Carbone A, Gloghini A, Gaidano G. Is plasmablastic lymphoma of the oral cavity an HHV-8-associated disease? Am J Surg Pathol. 2004; 28:1251–1252. PMID: 15316328.

13. Cioc AM, Allen C, Kalmar JR, Suster S, Baiocchi R, Nuovo GJ. Oral plasmablastic lymphomas in AIDS patients are associated with human herpesvirus 8. Am J Surg Pathol. 2004; 28:41–46. PMID: 14707862.
14. Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008; 83:804–809. PMID: 18756521.

Fig. 1
Abdominal CT data. (A) Coronal view of abdominal CT showing an anal fistula with 2 cm abscess formation (arrow). (B) Axial view of abdominal CT showing the anal fistula (arrow).
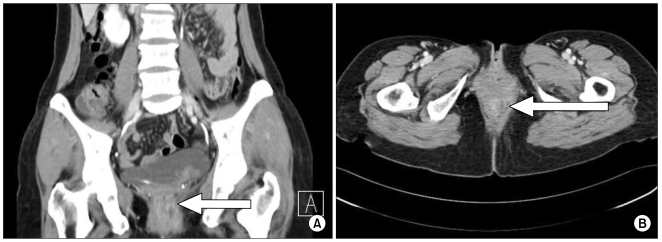
Fig. 2
PBL histological and immunohistochemical findings including proliferation index. (A) Hematoxylin and eosin stained section displaying large atypical cells with abundant cytoplasm. (B) Tumor cells were strongly positive in a diffuse fashion for CD138. (C) In situ hybridization for EBV-encoded RNA was positive in the neoplastic cells. (D) The proliferation index, as determined by Ki-67, was higher than 95%. (magnification, ×40).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download