Abstract
Purpose
Heptaplatin (Sunpla) is a cisplatin derivative. A phase IIb trial using heptaplatin resulted in a 34% response rate with mild nephrotoxicity. We conducted a randomized phase III trial of heptaplatin plus 5-FU compared with cisplatin plus 5-FU in patients with advanced gastric cancer.
Materials and Methods
One hundred seventy-four patients (heptaplatin, n=88; cisplatin, n=86) from 13 centers were enrolled. The eligibility criteria were as follows: patients with pathologically-proven adenocarcinoma, chemonaive patients, or patients who had received only single adjuvant chemotherapy, and who had a measurable or evaluable lesion. On day 1, heptaplatin (400 mg/m2) or cisplatin (60 mg/m2) was given over 1 hour with 5-FU (1 gm/m2) on days 1~5 every 4 weeks.
Results
At the time of survival analysis, the median overall survival was 7.3 months in the 5-FU + heptaplatin (FH) arm and 7.9 months in the 5-FU + cisplatin (FP) arm (p=0.24). Of the FH patients, 34.2% (complete response [CR], 1.3%; partial response [PR], 32.9%) experienced a confirmed objective response compared with 35.9% (CR 0%, PR 35.9%) of FP patients (p=0.78). The median-time-to-progression was 2.5 months in the FH arm and 2.3 months in the FP arm. The incidence of neutropenia was higher with FP (28%) than with FH (16%; p=0.06); grade 3~4 nausea and vomiting were more frequent in the FP than in the FH arm (p=0.01 and p=0.05, respectively). The incidence of increased proteinuria and creatininemia was higher with FH than with FP; however, there was no statistical difference. There were no treatment-related deaths.
Go to : 
Despite the reduction in the incidence of gastric cancer in most areas of the world, gastric cancer remains the second leading cause of cancer deaths worldwide (1,2). In Korea, surgery is still the most effective treatment for gastric cancer and good survival can be achieved if the tumor is resectable. In contrast, unresectable, advanced, or recurrent gastric cancer has a poor prognosis and chemotherapy is the most important treatment for prolongation of survival. To date, combination chemotherapy with 5-fluorouracil (5-FU) and cisplatin has been used most widely for such cases. This two-drug regimen has a superior response rate in comparison with single agent 5-FU. Indeed, the combination regimen has response rates ranging from 10% to 35%, and median survival times from 6~8 months, with approximately 10% of patients surviving 2~4 years (3,4).
Even though many oncologists agree with the effectiveness of cisplatin against gastric carcinoma, there are two major problems with this agent. First, cancer cells show primary or acquired resistance to cisplatin (5). Second, significant side effects are observed, such as severe nausea and vomiting, nephrotoxicity, and neurotoxicity (6).
To overcome these drawbacks of cisplatin, extensive efforts have been made to develop new cisplatin analogs with equivalent or greater antitumor activity and lower toxicity (7,8). Among the cisplatin analogs, carboplatin has reduced renal and gastrointestinal toxicities as compared with cisplatin (9) however, carboplatin has no enhanced therapeutic efficacy over cisplatin and has not circumvented the acquired resistance to cisplatin due to its cross-resistance (10). Heptaplatin (cis-malonatol, {[4R, 5R]-4, 5 bis caminomethyl}-2 - isopropyl- 1, 3-dioxolane platinum [II], SKI-2053R, Sunpla®; SK Chemicals, Korea) is a new platinum derivative. In vitro studies have shown that heptaplatin has high antitumor activity against various cancer cell lines (11-13). A phase II study showed that this agent has a response rate of 17% as a single agent and a response rate of 21% in combination with 5-FU in advanced gastric cancer (14,15).
The purpose of this clinical trial was to determine the difference between 5-FU + heptaplatin (FH) and 5-FU + cisplatin (FP) regimens in terms of efficacy and toxicity in the treatment of advanced gastric cancer.
Go to : 
This study was approved by the institutional review boards of the individual centers selected for this study. Written informed consent was obtained from all patients prior to the initiation of therapy. The study was designed to enroll patients with unresectable, pathologically-proven, locally advanced and/or metastatic adenocarcinoma of the stomach. Other eligibility criteria included the presence of measurable or evaluable disease, age between 20 and 70 years, ECOG 0-2, prior adjuvant chemotherapy without cisplatin if completed 30 days before randomization, surgery 2 weeks or longer before randomization, life expectancy ≥ 3 months, adequate biological parameters (i.e., WBC count > 4,000/mm3; platelet count > 100,000/mm3; aspartate aminotransferase [AST] and alanine aminotransferase [ALT] ≤ 2.0× the institutional upper limits of normal [ULN]; bilirubin ≤ 2.0 mg/dl; creatinine ≤ 1.2 mg/dl; and creatinine clearance ≥ 60 ml/min), and no serious cardiopulmonary co-morbidity that could impair participation in the study. Exclusion criteria included prior systemic chemotherapy, pregnancy or lactation, uncontrolled infection, chronic debilitating disease, metastasis to the central nervous system, psychiatric disorders, prior treatment with platinum derivatives, and hypersensitivity to cisplatin, carboplatin, other platinum derivatives, or mannitol.
Patients randomized to the treatment arm (FH) received heptaplatin (400 mg/m2) as an l-hour continuous infusion on day 1, followed by 5-FU (1,000 mg/m2/day) as a 12-hour continuous infusion on days 1~5 every 4 weeks. In the control arm (FP), patients received cisplatin (60 mg/m2) as an l-hour infusion on day 1, followed by 5-FU (1,000 mg/m2/day) as a 12-hour continuous infusion on days 1~5 every 4 weeks. Treatment was administered until disease progression, unacceptable toxicity, or withdrawal of consent.
All patients received antiemetic prophylaxis with intravenous ondansetron, and pre- and post-hydration. Granulocyte colony-stimulating factor was recommended for febrile neutropenia, neutropenic infection, or grade 3~4 nentropenia.
A dose reduction was made on the basis of the worst toxicity observed during the previous cycle. In cases of grade 3~4 neutropenia, thrombocytopenia, mucositis, and hepatotoxicity, the subsequent cycle was administered after recovery with a 25% reduction of the platinum and 5-FU. Also, a 50% reduction of the platinum was made when the creatinine clearance ranged between 40 and 60 ml/min. Chemotherapy cycles were delayed up to 2 weeks for recovery from neutropenia ≥ grade 2 or any episodes of thrombocytopenia or mucositis. Patients were disenrolled from the study if they failed to recover after a 2-week delay, needed more than a two dose reduction, or had a creatinine level ≥ 2.5 mg/dl.
A medical history was obtained and a physical examination was performed prior to enrollment. An ECG, chest x-ray, and biological analyses (CBC count; serum creatinine, creatinine clearance, bilirubin, AST, ALT; alkaline phosphatase, Ca++, Mg++, PO4-, Na+, K+, Cl-; urine analysis) were performed within the 2-week period before the initiation of treatment. Assessment of target lesions by computed tomography (CT) was performed within 14 days of the start of treatment. During the treatment period, a blood count, evaluation of toxicity, and a physical examination were performed before each cycle of chemotherapy. Assessment of target lesions was made by the same imaging method every two cycles of chemotherapy, as defined by WHO criteria (22); objective responses were confirmed by imaging 1 month after completion of each 2nd cycle by the participating investigators.
At the end of every two cycles, each patient's response was assigned to one of the following categories: complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), early progression, and death from any cause. Time-to-progression (TTP) was calculated from the first treatment infusion to the first objective evidence of disease progression, as assessed by CT scan. Overall survival (OS) was measured from initial treatment until death.
The National Cancer Institute-Common Toxicity Criteria (NCI-CTC), version 2.0, was used for adverse event reporting. Toxicity was evaluated prior to each cycle. The maximum grade for each type of toxicity was recorded for each patient and the frequency tables were used to determine toxicity patterns. If a patient's toxicity persisted after the termination of chemotherapy, the patient was followed-up until full recovery from the toxicity. If feasible, patients were followed-up every 3 months after treatment until death.
An intention-to-treat analysis was used and included all patients who satisfied the inclusion and exclusion criteria, and voluntarily signed the informed consent form. The primary endpoint of this phase III study was to detect an equivalent OS for the FH treatment arm compared to the FP control arm. The secondary endpoints were the response rate, the duration of response, and the TTP. These parameters were analyzed after at least 2 cycles in patients with an objective response, or after 1 cycle in patients with progressive disease. The safety analysis was done in all patients receiving at least 1 cycle. The sample size was calculated to ensure that the study had a power of 90% to prove the equivalence in OS of 37 weeks for FP based on the previous study with a two-sided 5% significance level using an unadjusted log-rank test; 81 patients in each arm were needed.
Randomization was carried out by a central coordinator, and stratification was applied according to performance status (0, 1, and 2), prior surgery (yes vs. no), and prior chemotherapy (yes vs. no). Patients were randomized to either the FH or FP arms according to the block randomization generated by a SAS program. Log-rank tests and Kaplan-Meier estimations were performed for the analysis of both TTP and OS. Objective responses were calculated with 95% confidence intervals (CIs). When suitable, χ2 or Fisher's exact test were used to compare qualitative data. Differences were assumed to be significant when p<0.05. No interim analysis was performed.
Go to : 
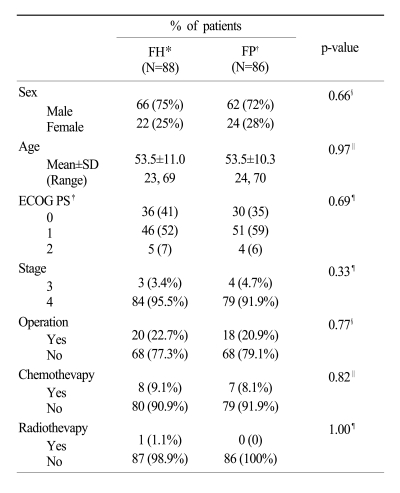
One hundred eighty-four patients from 13 centers were enrolled in this study between July 2000 and January 2004. Of the 184 patients, 174 patients (88 in the FH arm and 86 in the FP arm) were considered evaluable. Ten patients (4 in the FH arm and 6 in the FP arm) were disenrolled due to consent withdrawn (5), protocol violations (4), and disease progression before treatment (1). Patient characteristics were well-balanced between both arms (Table 1). The median age was 53 years (range, 23~70 years), and the ECOG 0~1 category was well-distributed (93% in the FH arm and 94% in the FP arm). Almost all patients had metastatic disease at randomization (97% in the FP arm and 93% in the FP arm). Only 8.6% of patients had prior chemotherapy (9.1% in the FH arm and 8.1% in the FP arm).
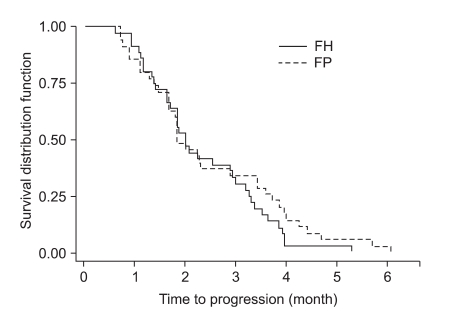
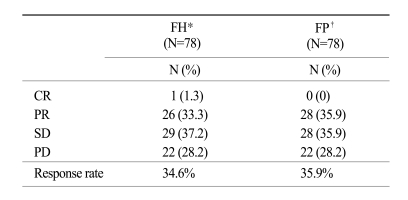
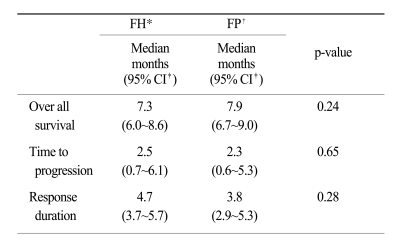
The tumor response rate was evaluated by the investigators. The median number of cycles was 3 for the FH arm and 4 for the FP arm. On the intent-to-treatment analysis, 34.2% (CR, 1.3%; PR, 32.9%) had a confirmed objective response in the FH patients, compared with 35.9% (CR, 0%; PR, 35.9%) in the FP patients (p=0.78; Table 2). The median OS was 7.3 months (95% CI, 6.0~8.6 months) in the FH arm and 7.9 months (95% CI, 6.8~9.0 months) in the FP arm; there was no significant difference between the treatment arms (p=0.24; Fig. 1). The median TTP was 2.5 months (range, 0.7~6.1 months) in the FH arm and 2.3 months (range, 0.6~5.3 months) in the FP arm (Fig. 2). The median response duration was 4.7 months (range, 3.7~5.7 months) in the FH arm and 3.8 months (range, 2.9~5.3 months) in the FP arm (Table 3).
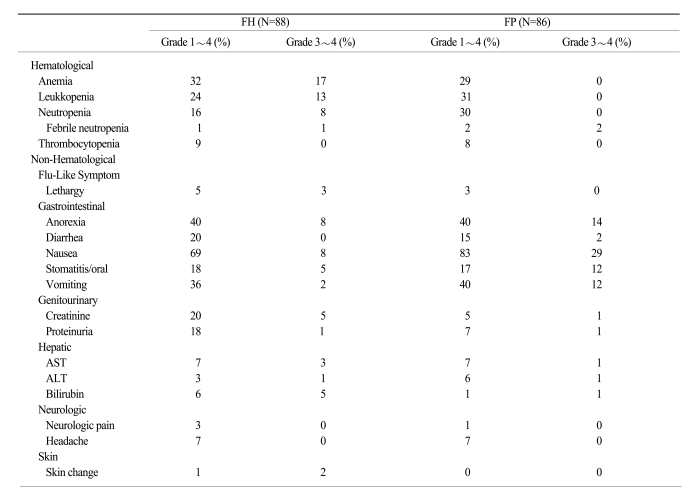
Six hundred twenty-four cycles were administered to 174 patients (313 cycles in the FH arm and 311 cycles in the FP arm). Toxicity, as assessed according to the NCI-CTC criteria, was generally mild, and hematologic and non-hematologic toxicities are summarized in Table 2. Almost all patients received full doses of both drugs. Dose intensities in the FH and FP arms were 97.9% and 99.2%, respectively.
Dose reductions were required in 6.4% and 3.9% of the patients in the FH and FP arms, respectively (p=0.15). Chemotherapy delays and/or discontinuations were necessary in < 20% of chemotherapy cycles (12% in the FH arm and 19% in the FP arm; p=0.02). The most common cause of delay was neutropenia. Anemia was the most common hematologic toxicity in both arms (32% in the FH arm and 30% in the FP arm). The incidence of neutropenia was higher in the FP arm (30%) than in the FH arm (16%; p=0.06). The incidence of grade 3~4 hematologic toxicities did not differ between the arms. Nausea was the most common non-hematologic toxicity in both arms (69% in the FH arm and 83% in the FP arm). Grade 3~4 nausea and vomiting were more frequent in the FP arm than in the FH arm (p=0.01 and p=0.05, respectively). The incidences of proteinuria and increased creatinine level were higher in the FH arm (18% and 15%, respectively) than the FP arm (7% and 5%, respectively). However, the incidences of grade 3~4 proteinuria and creatinine level increase were not significantly different between the arms. The frequencies of non-hematologic toxicities, such as neurotoxicity and hepatotoxicity, were similar in both arms. One death occurred in a patient not responding to therapy, but this death was deemed unrelated to treatment (Table 4).
Go to : 
Heptaplatin (Sunpla®) is a third generation anticancer platinum complex that has a broader spectrum of anticancer activity, insignificant nephrotoxicity at the optimal dose, and suitable physicochemical properties, such as high solubility (11). Preclinical studies on heptaplatin have shown that its antitumor effect on various cancer cell lines was comparable with or superior to that of cisplatin (17). Although cisplatin is one of the most potent anticancer drugs for a variety of human cancers, undesirable effects, such as severe nephrotoxicity, high emetogenicity, and neurotoxicity, along with the development of drug resistance, have limited its clinical usefulness (18,19).
Many combination chemotherapy regimens for the treatment of advanced gastric cancer have been developed and have been shown to have high response rates (20-22). However, a standard chemotherapeutic regimen has not been established because there have been no results from a randomized phase III trial showing a survival benefit when compared with 5-FU alone. In the study of Kim et al. study (3), which compared FP vs. 5-FU, adriamycin, and mitomycin (FAM) vs. 5-FU, the FP group had a 51% objective response rate and a trend to an increase in the median survival. For all these reasons, FP is preferred as the reference regimen by many clinicians.
Kim et al (14). reported a clinical phase II trial involving SK-2053R as a single agent in the treatment of patients with advanced gastric adenocarcinoma. The response rate was 17% and the median duration of response was 7.2 months. Patients tolerated the treatment without significant toxicity (14). After these results, combination chemotherapy with 5-FU and heptaplatin as first-line treatment in patients with advanced gastric cancer was administered (15). This study, based on a continuous infusion of 5-FU with heptaplatin, demonstrated a response rate of 21% and a median PFS and OS of 1.9 and 6.2 months, respectively. However, because 36% of the patients were not assessable for response in the study, their results were not sufficient to lead to a verifiable conclusion regarding efficacy and toxicity. Thus, The Korean Cancer Study Group (KCSC) initiated the current randomized phase III study for comparison between FH and FP regimens in patients with advanced gastric cancer.
In the current study, the median OS was 7.3 months in the FH arm and 7.9 months in the FP arm. On the intent-to-treatment analysis, 34.2% of the FH patients showed an objective response compared with 35.9% of the FP patients. The median TTP was 2.5 months in the FH arm and 2.3 months in the FP arm.
The FP arm in the present trial yielded results comparable with another phase III trial of cisplatin combined with 5-FU (4), which supports the conclusion that FH is comparable to FP in this treatment setting based on the consistently similar efficacy results. Nevertheless, the significance of a longer TTP in the other study is difficult to compare with the current study due to the higher incidence of esophageal or esophagogastric disease (43%) and the proportion of locally advanced disease (37%) in other study (i.e., nearly all patients had gastric adenocarcinoma and metastatic disease at randomization [97% in the FH arm and 93% in the FP arm]).
The efficacy of anticancer drugs may depend on the schedule of administration as well as the delivered dose. Most anticancer agents, if not all, can efficiently kill tumor cells when in an active phase of the cell cycle. In general, solid tumors are composed mostly of cells that are in the quiescent phase. It is therefore conceivable that prolonged infusion of a cytotoxic agent allows better drug exposure to tumor cells, which subsequently enter the cell cycle, as opposed to short-term infusional treatment, which allows only a small proportion of cells in an active phase of the cycle at the point to be affected. Since the protein-unbound fraction exerts the anticancer effects of platinum-based anticancer drug, exposure of more tumor cells to an adequate level of the free drug by extension of the exposure time could theoretically produce a great cell kill (23). The peak levels of ultrafiltrable platinum from SKI-2053R measured after a 24-hour infusion were only about 12-fold lower than those obtained following a 1-hour infusion. It is very promising that a steady state for a relatively high level of free platinum is maintained during long-term (12- and 24-hours) infusion shortly after the start of the infusion (1~3 hours). Since free platinum is the major component possessing antitumor activity in platinum-based anticancer drugs (24), the prolonged steady state achieved for free platinum from SKI-2053R could allow more tumor cells in the active phase of the cell cycle to be exposed to the drug in vivo.
Overall, the FH arm displayed a similar safety profile with the FP, characterized by no treatment-related deaths and a low rate of discontinuations due to toxicity. The incidence of neutropenia was higher with the FP than with the FH, yet the incidence of grade 3~4 hematologic toxicity did not differ between the two arms. Nausea was the most common non-hematologic toxicity in both arms. Grade 3~4 nausea and vomiting were more frequent in the FP arm than the FH arm. Ahn et al.(25) reported nephrotoxicity of heptaplatin in a randomized comparison with cisplatin in advanced gastric cancer. The 24-hour proteinuria on day 5 was markedly increased in the heptaplatin arm than the cisplatin arm, and the creatinine clearance showed a greater decrease in the heptaplatin arm than the cisplatin arm. The differences in these parameters between the two arms were statistically significant throughout the subsequent cycles. In the current study, the incidence of proteinuria was higher with the FH than with the FP, however, the incidence of grade 3~4 proteinuria and creatinine level increase were not significantly different in both arms. Further efforts to detect and minimize the nephrotoxicity of heptaplatin are clearly warranted.
Go to : 
Heptaplatin showed similar effects to cisplatin when combined with 5-FU in advanced gastric cancer patients with tolerable toxicities. Based on the results of the pharmacologic and pharmacokinetic findings of SKI-2053R, the therapeutic efficacy of SKI-2053R given by continuous long-term infusion should be investigated in future clinical studies.
Go to : 
Acknowledgement
The authors thank SK Chemical for study coordination. Heptaplatin was provided by SK Chemical of Korea.
Go to : 
References
1. Jemal A, Thomas A, Murray T, Thun M. Cancer statistics 2002. CA Cancer J Clin. 2002; 52:23–47. PMID: 11814064.

2. Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics 2001. CA Cancer J Clin. 2001; 51:15–36. PMID: 11577478.

3. Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, et al. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993; 71:3813–3818. PMID: 8508349.

4. Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol. 2003; 21:54–59. PMID: 12506170.

5. Fram RJ. Cisplatin and platinum analogues: recent advances. Curr Opin Oncol. 1992; 4:1073–1079. PMID: 1457521.
6. Reed E, Dabholkar M, Chabner BA. Chabner BA, Longo DL, editors. Platinum analogues. Cancer chemotherapy and biotherapy: principles and practice. 1996. 2nd. edition. Philadelphia: Lippincott-Raven;p. 357–378.
7. Harrap KR. Preclinical studies identifying carboplatin as a visible cisplatin alternative. Cancer Treat Rev. 1985; 12(Suppl A):21–33. PMID: 3910219.
8. McKeage MJ, Higgins JD 3rd, Kelland LR. Platinum and other metal coordination compounds in cancer chemotherapy. A commentary on the sixth international symposium, San Diego, Califonia, 23-26th January 1991. Br J Cancer. 1991; 64:788–792. PMID: 1911229.
9. Rose WC, Schuring JE. Preclinical antitumor and toxicologic profile of carboplatin. Cancer Treat Rev. 1985; 12(Suppl A):1–19. PMID: 3910215.

10. Gore ME, Fryatt I, Wiltshaw E, Dawson T, Robinson BA, Calvert AH. Cisplatin/carboplatin cross-resistance in ovarian cancer. Br J Cancer. 1989; 60:767–769. PMID: 2803953.

11. Kim DK, Kim G, Gam J, Cho YB, Kim HT, Tai JH, et al. Synthesis and antitumor activity of a series of [2-sub-stituted-4, 5-bis (aminomethl)-1, 3-dioxolane] platinum(II) complexes. J Med Chem. 1994; 37:1471–1485. PMID: 8182706.
12. Kim DK, Kim HT, Cho YB, Tai JH, Ahn JS, Kim TS, et al. Antitumor activity of cis-malonato [(4R, 5R)-4, 5 bis(aminomethy)-2-isopropyl-1,3-dioxolane] platinum(II), a new platinum analogue, as an anticancer agent. Cancer Chemother Pharmacol. 1995; 35:441–445. PMID: 7850928.
13. Kim HT, Kim DK, Cho YB, Kim TS, Jung I, Kim KH, et al. Influence of exposure and infusion times on the cytotoxicity and pharmacokinetics of cis-malonato [(4R, 5R)-4,5-bis(aminomethy)-2-isopropyl-1,3-dioxolane] platinum(II). Cancer Chemother Pharmacol. 1998; 41:109–116. PMID: 9443623.
14. Kim NK, Im SA, Kim DW, Lee MH, Jung CW, Cho EK, et al. Phase II clinical trial of SKI-2053R, a new platinum analog, in the treatment of patients with advanced gastric adenocarcinoma. Cancer. 1999; 86:1109–1115. PMID: 10506693.

15. Min YJ, Bang SJ, Shin JW, Kim DH, Park JH, Kim GY, et al. Combination chemotherapy with 5-fluorouracil and heptaplatin as first-line treatment in patients with advanced gastric cancer. J Korean Med Sci. 2004; 19:369–373. PMID: 15201502.

16. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47:207–214. PMID: 7459811.

17. Hong WS, Kim HT, Kim KH, Kim DK. In vitro antitumor activity of a new platinum complex, cis-malonato (4R,5R)-4,5-bis(aminomethyl)-1,3-dioxolane-platinum(II) (SKI2053R), against human lung and stomach cancer cell lines. Anticancer Res. 1995; 15:51–54. PMID: 7733640.
18. Loehrer PJ, Einhorn LH. Drugs five years later. Cisplatin. Ann Intern Med. 1984; 100:704–713. PMID: 6370067.
20. Moertel CG, Rubin J, O'Connell MJ, Schutt AJ, Wieand HS. A phase II study of combined 5-fluorouracil, doxorubicin, and cisplatin in the treatment of advanced upper gastrointestinal adenocarcinomas. J Clin Oncol. 1986; 4:1053–1057. PMID: 3014083.

21. Kim YH. Chemotherapy for advanced gastric cancer: slow but further progression. Cancer Res Treat. 2005; 37:79–86.
22. Ohtsu A, Shimada Y, Yoshida S, Saito H, Seki S, Morise K, et al. Phase II study of protracted infusional 5-flurouracil combined with cisplatinum for advanced gastric cancer: report from the Japan Clinical Oncoloty Group (JCOG). Eur J Cancer. 1994; 30A:2091–2093. PMID: 7857709.
23. Gormley PE, Bull JM, Leroy AF, Cysyk R. Kinetics of cis-dichlorodiammineplatinum. Clin Pharmacol Ther. 1979; 25:351–357. PMID: 761445.
24. Kim DK, Kim HT, Tai JH, Cho YB, Kim TS, Kim KH, et al. Pharmacokinetics and antitumor activity of a new platinum compound, cis-malonato[(4R,5R)-4,5-bis(aminomethy1)-2isopropy1-1,3-ioxolane]platinum(II) as determined by ex vivo pharmacodynamics. Cancer Chemother pharmacol. 1995; 37:1–6. PMID: 7497577.
25. Ahn JH, Kang YK, Kim TW, Bang H, Chang HM, Kang WC, et al. Nephrotoxicity of heptaplatin: a randomized comparion with cisplatin in advanced gastric cancer. Cancer Chemother Pharmacol. 2002; 50:104–110. PMID: 12172973.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download