Abstract
Purpose
Although multiple primary colorectal cancer has been recognized as a significant clinical entity, its clinical and pathological features and its prognosis are still controversial. The purpose of this study was to clarify clinical and pathological features of multiple primary colorectal cancer.
Materials and Methods
Among 1669 patients who underwent surgery for primary colorectal cancer from January 1997 to June 2005, 26 patients (1.6%) with multiple primary colorectal cancer were identified. We reviewed clinical characteristics including diagnostic interval, lesions, operating methods, and TNM stage, and we defined the index lesion as the most advanced lesion among the synchronous lesions. For the purposes of the study, the colon and rectum were classified into three segments. The right-side colon included the appendix, cecum, ascending colon, hepatic flexure, and transverse colon, and the left-side colon included the splenic flexure, descending colon, and sigmoid colon.
Results
Of the 26 patients with multiple primary colorectal cancers, nineteen patients were male and seven patients were female, with a mean age of 61.5 years. Nineteen patients had synchronous colorectal cancers and seven patients had metachronous colorectal cancers. In the metachronous cases, the mean diagnostic interval was 36.8 months. The site of the first lesion in metachronous colorectal cancers was the right colon in five cases (71.4%) and the left colon in two cases (28.6%), and the site of the second lesion was the rectum in six cases (55.5%), the right colon in three cases (33.3%), and the left colon in one case. The TNM stage of the second lesions in the metachronous colorectal cancers was stage II in four cases (57.1%), stage III in one case (14.3%), and stage IV in one case (14.3%). For the synchronous colorectal cancers, the operation methods were single-segment resection combined with endoscopic mucosal resection in five cases (26.3%), single-segment resection alone in six cases, two-segment resection in six cases, and total colectomy in two cases.
Conclusion
In metachronous colorectal cancers, the secondary lesions were later-stage cancer. Therefore, careful postoperative follow-up is necessary for patients who have undergone surgery for colorectal cancers. Further study of therapeutic modalities is important for synchronous colorectal cancers.
Multiple primary cancer is defined as two or more cancers detected in the same or other organs of an individual patient, either synchronously or metachronously. Each tumor is independent rather than a metastasis from another tumor. The incidence of multiple primary cancers identified in the colon and rectum is about 2~5% (1~7). This incidence increases to 10~20% in patients with familiar adenomatous polyposis, hereditary non-polyposis, colorectal cancer, and ulcerative colitis.
Preoperative detection of synchronous colorectal cancer is very important to planning treatment. If synchronous colorectal cancers are detected during an operation, the surgical procedure occasionally needs to be altered. Non-detected synchronous colorectal cancers usually ultimately present as early metachronous carcinoma of advanced stages and generally require re-operation (8). The purpose of the present study was to evaluate the clinical characteristics of synchronous and metachronous colorectal cancer.
From January 1997 to June 2005, 1,669 cases of invasive colorectal adenocarcinoma were surgically resected at our institute. Of these, 1,643 cases were single carcinomas and 26 cases (1.6%) were multiple primary colorectal cancers as evaluated by the Moertel criteria (9). Patients with familiar polyposis adenomatosis, hereditary nonpolyposis, colorectal cancer, and ulcerative colitis were excluded. Preoperative evaluation was conducted by barium contrast enema and/or colonoscopy, and CT scans and blood tumor markers were ordinarily employed. Synchronous colorectal cancers were defined as tumors diagnosed either preoperatively, during an operation by palpation, or postoperatively by colonoscopy within a period less than 6 months, and at least 4 cm distant from each other.
In synchronous colorectal cancer, the index lesions were defined to be the tumors that were the most advanced pathologically. When two or more lesions were at an identical pathological stage, the largest lesion was regarded as the index lesion and the other lesions were designated as the concurrent lesions (8). Cancers detected more than six months after the prior operation were regarded as metachronous colorectal cancers (9,10). Anastomotic recurrence and metastasis were excluded. In metachronous colorectal cancer, the carcinoma diagnosed at the prior operation was regarded as the first lesion.
Tumor locations were classified into three groups as being in the right-side colon, left-side colon, or rectum. The right-side colon included the appendix, cecum, ascending colon, hepatic flexure colon, and transverse colon. The left-side colon included the splenic flexure colon, descending colon, and sigmoid colon (8).
We retrospectively reviewed the medical records of 26 patients with multiple primary colorectal cancers. Tumor stages were determined according to TNM classification (American Joint Committee on Cancer, 6th ed.).
Among 26 patients with multiple primary colorectal cancers, synchronous colorectal cancers were found in nineteen cases. All cases were identified by preoperative colonoscopy. The number of synchronous lesions was two in most patients, but one patient had three synchronous lesions, with descending colon cancer as the index lesion together with cecal cancer and rectal cancer. The index site in the synchronous colorectal cancers was the rectum in nine cases (47.4%), the right-side colon in six cases (31.6%), and the left-side colon in four cases (21.1%) (Table 1).
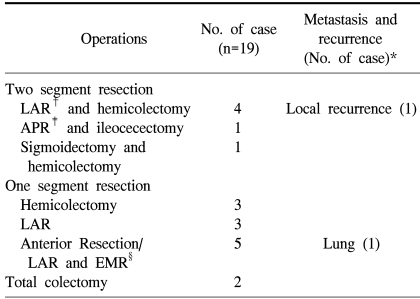
Single-segment resections, such as low anterior resection or right/left hemicolectomy, were performed in six patients (31.6%), and two-segment resection of the colon for each synchronous lesion was performed in another six patients (31.6%). In five patients (26.3%), a combination of single-segment resection for the index lesions and endoscopic mucosal resection for the concurrent lesions was performed, and in two patients total colectomy was done. One patient had three carcinomas, with lesions in the cecum, the descending colon, and the rectum. The other patients had carcinomas in the transverse colon and in the rectum (Table 2).
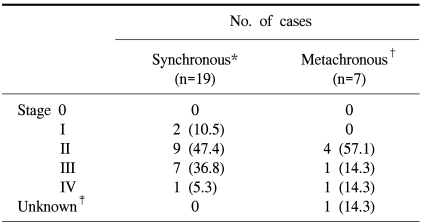
The TNM stage of the index lesion was stage I in two cases (10.5%), stage II in nine cases (47.4%), stage III in seven cases (36.8%), and stage IV in one case (5.3%) (Table 3). The mean follow-up time for synchronous colorectal cancer was 34.6 months (1~62 months). In two patients, recurrences were identified. One patient had a local recurrence 33 after months after a two-segment resection for synchronous lesions, and the other patient had pulmonary metastasis 22 months after single-segment resection for the index lesion and endoscopic mucosal resection for the concurrent lesion. These two patients underwent total colectomy, and no recurrence was identified with 43 and 50 months of follow-up.
Among the 26 patients with multiple primary colorectal cancers, metachronous colorectal cancers were identified in seven. The mean time of diagnostic interval between the first cancer and the second cancer was 36.8 months (28~49 months). In two patients, a third cancer was identified. One patient had a sigmoid colon cancer as the primary cancer, followed by descending colon cancer as the second cancer after 38 months, and cecal cancer as the third cancer 24 months after the second operation. The other patient had transverse colon cancer as the primary cancer, with cecal cancer and rectal cancer synchronously identified 38 months later. The site of the first cancer was the right colon in five cases (71.4%) and the left colon in two cases (28.6%). The second cancer was identified in the rectum in six cases (55.5%), the right colon in three cases (33.3%), and the left colon in one case (Table 1). The TNM stage of the second cancer was stage II in four cases (57.1%), stage III in one case (14.3%), and stage IV in one case (14.3%) (Table 3).
Due to remarkable improvement in cancer treatment, many cancer patients survive long after treatment. Consequently, they are at increased risk of developing multiple primary cancers. Ueno et al(5) reported that the risk of developing multiple primary colorectal cancers was the second highest among all cancers. The incidence of multiple primary cancers identified in the colon and rectum is about 2~5% (1~7), and in elderly patients with colorectal cancer the incidence of multiple primary colorectal cancer is increased (11). Multiple primary colorectal cancer is more predominant in males (9). Although the most common sites of multiple primary colorectal cancers are the sigmoid colon and the rectum, identification of multiple primary cancers in the proximal colon has increased (12). This observed site predominance in multiple primary colorectal cancer is probably attributable to the fact that colorectal cancers frequently develop in the sigmoid colon and the rectum. Kim et al. (13) suggested that tumors in the sigmoid colon and the rectum can be easily detected with sigmoidoscopy. In the present study, there was no predominance seen in the site location of the multiple primary colorectal cancers.
Preoperative identification of synchronous colorectal cancers is important to planning treatment, because the operative plan varies depending on the detection of synchronous colorectal cancers and the identification of tumor location. Although most synchronous colorectal cancers can be detected by a preoperative barium enema or colonoscopy, tumors are still often missed.
Finan et al(14) reported that synchronous colorectal cancers in 58% of 52 patients were missed at pre-operative evaluation. In their study, 24% of synchronous colorectal cancers were identified by intra-operative evaluation and 34% were identified incidentally from the post-operative specimen. In a barium enema test, lesions can be missed because of several reasons, most usually colonic bleeding and poor bowel cleansing.
The identification of tumors in the proximal colon by colonoscopy can be limited by obstruction of lesions in the distal colon. If pre-operative evaluation of the whole colon is limited, intra-operative palpation or intra-operative colonoscopy are essential (15~16). Recently, virtual colonoscopy has become a viable alternative method for evaluation of the whole colon (17). All of our cases were identified using pre-operative colonoscopy.
In metachronous colorectal cancers, the diagnostic interval between the first and second cancers ranges between eight months and twenty years (7), with a mean time of 6~8 years (13,18). In the present study, the mean time of diagnostic interval was 36.8 months.
Synchronous colorectal cancers involving index lesions and concurrent lesions tend to be less advanced than single colorectal cancers (4,6,13,14,19), leading to the common definition of concurrent lesions as less advanced lesions. In metachronous colorectal cancers, second cancers are also typically less advanced, with TNM stage I in 31% of cancers and stage II in 66.6% (7). In the present study, stage II lesions were found in 57.1% of metachronous cancer cases. Patients found to have stage II second cancers were those who were given follow-up colonoscopy one, three, and five years after the first operation. Patients with stages III and IV cancer were patients who did not receive serial postoperative colonoscopic evaluation or who underwent previous operation for their first lesions at other institutes.
The five-year survival rate for multiple primary colorectal cancers was similar to that of single colorectal cancers in one previous study (4), and in other studies the survival with multiple primary colorectal cancer was somewhat worse than for single colorectal cancers but without statistical significance (8,13,20). When concurrent cancers are not detected, they are subsequently diagnosed as metachronous cancer with advanced lesions (8). In synchronous colorectal cancer, because preoperative identification of concurrent lesions is vital to improved prognosis, careful colonoscopic evaluation of the whole colon is necessary (21~22). For adequate management of multiple primary colorectal cancers, subtotal or total colectomy may occasionally be necessary (23). Colonoscopy surveillance at six-month intervals for 24~48 months after an operations is recommended in order to achieve early detection of metachronous colorectal cancer (7). Further study is need to evaluate the predictive value of MSI (microsatellite instability) and mismatch-repair genes in multiple primary colorectal cancer (24~25).
In the current study, most second lesions in metachrnous colorectal cancer were identified 3~5 years after operation for the initial lesions. We recommend further study for evaluation of clinical characteristics, diagnostic interval, and predictive factors for metachronous colorectal cancers. For synchronous colorectal cancer, additional study is needed to elucidate proper management in relation to the size, stage, and location of the concurrent lesions.
References
1. Welch JP. Multiple colorectal tumors. An appraisal of natural history and therapeutic options. Am J Surg. 1981; 142:274–280. PMID: 7258541.
2. Cunliffe WJ, Hasleton PS, Tweedle DE, Schofield PF. Incidence of synchronous and metachronous colorectal carcinoma. Br J Surg. 1984; 71:941–943. PMID: 6498470.

3. Takeuchi H, Toda T, Nagasaki S, Kawano T, Minamisono Y, Maehara Y, et al. Synchronous multiple colorectal adenocarcinoma. J Surg Oncol. 1997; 64:304–307. PMID: 9142187.
4. Chen HS, Sheen-Chen SM. Synchronous and "early" metachronous colorectal adenocarcinoma. Dis Colon Rectum. 2000; 43:1093–1099. PMID: 10950007.

5. Ueno M, Muto T, Oya M, Ota H, Azekura K, Yamaguchi T. Multiple primary cancer: an experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int J Clin Oncol. 2003; 8:162–167. PMID: 12851840.

6. Wang HZ, Huang XF, Wang Y, Ji JF, Gu J. Clinical features, diagnosis, treatment and prognosis of multiple primary colorectal carcinoma. World J Gastroenterol. 2004; 15:2136–2139. PMID: 15237453.

7. Papadopoulos V, Michalopoulos A, Basdanis G, Papapolychroniadis K, Paramythiotis D, Fotiadis P, et al. Synchronous and metachronous colorectal carcinoma. Tech Coloproctol. 2004; 8:S97–S100. PMID: 15655657.

8. Oya M, Takahashi S, Okuyama T, Yamaguchi M, Ueda Y. Synchronous colorectal carcinoma: clinico-pathological features and prognosis. Jpn J Clin Oncol. 2003; 33:38–43. PMID: 12604723.

9. Moertel CG, Bargen JA, Dockerty MB. Multiple carcinoma of the large intestine: a review of the literature and a study of 261 cases. Gastroenterology. 1958; 34:85–98. PMID: 13501357.
10. Evers BM, Mullins RJ, Matthews TH, Broghamer WL, Polk HC Jr. Multiple adenocarcinoma of the colon and rectum. An analysis of incidence and current trends. Dis Colon Rectum. 1988; 31:518–522. PMID: 3391060.
11. Arai T, Sawabe M, Takubo K, Kanazawa K, Esaki Y. Multiple colorectal cancers in the elderly: a retrospective study of both surgical and autopsy cases. J Gastroenterol. 2001; 36:748–752. PMID: 11757746.

12. Rennert G, Robinson E, Rennert HS, Neugut AI. Clinical characteristics of metachronous colorectal tumors. Int J Cancer. 1955; 60:743–747. PMID: 7896438.

13. Kim YJ, Kim NK, Lee KY, Sohn SK, Min JS. Clinicopathologic characteristics of multiple primary colorectal cancer. J Korean Soc Coloproctol. 2002; 18:343–348.
14. Finan PJ, Ritchie JK, Hawley PR. Synchronous and 'early' metachronous carcinoma of the colon and rectum. Br J Surg. 1987; 74:945–947. PMID: 3664228.
15. Kaibara N, Kimura O, Nishidoi H, Miyano Y, Koga S. Intraoperative colonoscopy for the diagnosis of multiple cancers of the large intestine. Jpn J Surg. 1982; 12:117–121. PMID: 7109357.

16. Barillari P, Ramacciato G, De Angelis P, Gozzo P, Indinnimeo M, Valabrega S, et al. Effect of preoperative colonoscopy on the incidence of synchronous and metachronous neoplasms. Acta Chir Scand. 1990; 156:163–166. PMID: 2330795.
17. Johnson CD, Hara AK, Reed JE. Computed tomographic colonoscopy (Virtual colonoscopy): a new method for detecting colorectal neoplasms. Endoscopy. 1997; 29:454–461. PMID: 9342563.
18. Heald RJ, Bussey HJ. Clinical experiences at St. Mark's Hospital with multiple synchronous cancers of the colon and rectum. Dis Colon Rectum. 1975; 18:6–10. PMID: 1126258.

19. Passman MA, Pommier RF, Vetto JT. Synchronous colon primaries have the same prognosis as solitary colon cancers. Dis Colon Rectum. 1996; 39:329–334. PMID: 8603557.

20. Kimura T, Iwagaki H, Fuchimoto S, Hizuta A, Orita K. Synchronous colorectal carcinoma. Hepatogastroenterology. 1994; 41:409–412. PMID: 7851846.
21. Pagana TJ, Ledesma EJ, Mittelman A, Nava HR. The use of colonoscopy in the study of synchronous colorectal neoplasms. Cancer. 1984; 53:356–359. PMID: 6690019.

22. Tate JJ, Rawlinson J, Royle GT, Brunton FJ, Taylor I. Pre-operative or postoperative colonic examination for synchronous lesions in colorectal cancer. Br J Surg. 1988; 75:1016–1018. PMID: 3219527.

23. Arenas RB, Fichera A, Mhoon D, Michelassi F. Incidence and therapeutic implications of synchronous colonic pathology in colorectal adenocarcinoma. Surgery. 1997; 122:706–710. PMID: 9347846.

24. Masubuchi S, Konishi F, Togashi K, Okamoto T, Senba S, Shitoh K, et al. The significance of microsatellite instability in predicting the development of metachronous multiple colorectal carcinomas in patients with nonfamilial colorectal carcinoma. Cancer. 1999; 85:1917–1924. PMID: 10223230.

25. Ericson K, Halvarsson B, Nagel J, Rambech E, Planck M, Piotrowska Z, et al. Defective mismatch-repair in patients with multiple primary tumours including colorectal cancer. Eur J Cancer. 2003; 39:240–248. PMID: 12509957.

Table 1
Characteristics of patients with multiple primary colorectal cancers
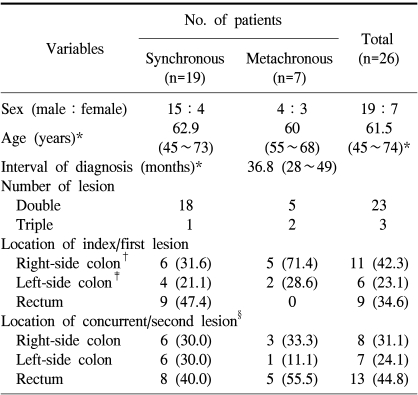
*values are mean (range); other values in parentheses are percentage. †right-side colon, which included appendix, cecum, ascending colon, hepatic flexure, and transverse colon, ‡left-side colon, which included splenic flexure, descending colon, and sigmoid colon, §number of concurrent synchronous lesion (n=20), and second metachronous lesion (n=9).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download