INTRODUCTION
MATERIALS AND METHODS
1) Materials
2) Mammalian Cell Culture and transient transfection
3) Cell growth assay
4) Preparation of Cell Lysates
5) Immunoprecipitation and in vitro Raf-1 kinase activity assay
RESULTS
1) The involvement of PKC in Raf-1 kinase activation
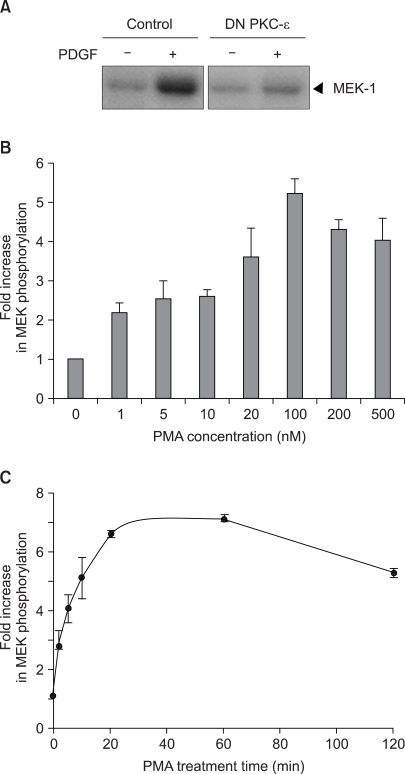 | Fig. 1The activation of Raf-1 protein kinase by PMA in the v-Ha-ras transfected NIH3T3 cells. In (A), the cells transfected with the mock control pMTH vector or with the pMTH vector encoding the dominant-negative PKC-ε were serum-deprived for 24 h in the presence of zinc acetate and then the cells were exposed to 20 ng/ml PDGF for 1 min. In (B) and (C), the subconfluent cells were serum-deprived for 24 h and then the cells were exposed to the indicated concentrations of PMA for 20 min (B), or to 100 nM PMA for the indicated times (C). In vitro Raf-1 kinase assays were performed on the immunoprecipitated Raf-1 proteins with using recombinant MEK as a substrate. In vitro Raf kinase assays were performed on the immunoprecipitated Raf-1 proteins with using recombinant MEK as substrate. The in vitro 32P-labeled MEK-1 protein was separated by 7.5% SDS-polyacrylamide gel electrophoresis and the phosphorylation was determined by autoradiography. In (B) and (C), the data is presented as the fold increase compared with the untreated cells. The values are the mean of three separate experiments with the error bar representing the standard deviations. |
2) The inhibitory effect of PMA on cell growth
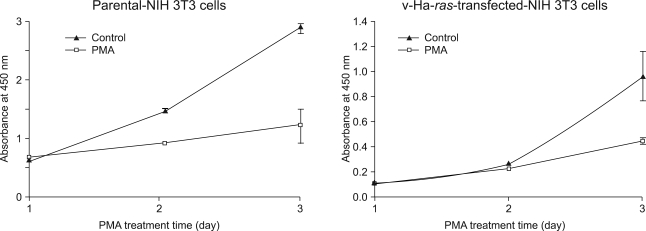 | Fig. 2Effect of PMA on the parental NIH 3T3 cells and its v-Ha-ras-transfected NIH 3T3 cells. The cells were treated with 100 nM PMA and then they were incubated in 96 well plates for 1, 2 or 3 days before the addition of the cell proliferation reagent WST-1. After an additional 4 h incubation period, the absorbance was measured by using an ELISA reader. The absorbance at 450 nm is expressed as the mean±SD of quadruplicate determinations from one of the three representative experiments. *p<0.01 as determined by Dunnett's T-test as compared to the vehicle control group. |
3) The effect of PI3 kinase inhibitor wortmannin on PMA-induced Raf-1 kinase activation
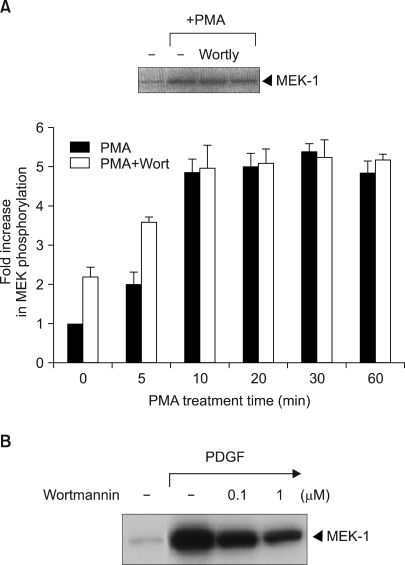 | Fig. 3The effect of the PI3 kinase inhibitor wortmannin or LY294002 on PMA-induced Raf-1 kinase activation. (A) The v-Ha-Ras transfected NIH 3T3 cells were serum-deprived for 24 h and then they were incubated in the absence or presence of 100 nM wortmannin (WORT) or 25 µM LY294002 (LY) for 30 min prior to stimulation with 100 nM PMA for either the indicated times or 20 min. The Raf-1 immunoprecipitates were assayed for kinase activity with using MEK-1 as the substrate. The data is presented as the fold increase compared with the untreated cells. The values are the mean of three separate experiments with the error bar representing the standard deviations. The figures of the inset show the results of typical experiments. (B) In vitro Raf-1 kinase assays were performed on the immunoprecipitated Raf-1 proteins from the parental NIH 3T3 cells that were treated with PDGF for 1 min in the absence or presence of 100 nM wortmannin. The in vitro 32P-labeled MEK-1 protein was separated by 7.5% SDS-polyacrylamide gel electrophoresis and the phosphorylation was determined by autoradiography. The results presented are all representative of three independent experiments. |
4) The role of reactive oxygen species in PMA-induced Raf-1 kinase activation
 | Fig. 4Effect of the antioxidant N-acetylcysteine on PMA-induced Raf-1 kinase activation. The v-Ha-Ras transfected NIH 3T3 cells were serum-deprived for 24 h and then they were incubated in the absence or presence of 20 mM N-acetylcysteine for 1 h prior to stimulation with 100 nM PMA (A) or 20 ng/ml PDGF (B) for either the indicated times or 20 min (in inset). The Raf-1 immunoprecipitates were assayed for kinase activity with using MEK-1 as a substrate. The data is presented as the fold increase compared with the untreated cells. The values are the mean of three separate experiments with the error bar representing the standard deviations. The figures of the inset show the results of typical experiments. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download