Abstract
Purpose
We investigated the immunoexpressions of cyclin D1, cyclin-dependent kinase inhibitor p16 and phosphorylated retinoblastoma (p-pRb) proteins in non-small cell lung carcinoma (NSCLC) to demonstrate their key roles in tumorigenesis, their relationship with the clinicopathologic factors, and their prognostic influences on the long-term survival.
Materials and Methods
115 surgically resected NSCLCs were immunohistochemically stained for the G1/S cell cycle proteins, with using a tissue microarray. The correlation between their immunoexpressions and the clinicopathologic prognostic factors, their inter-relationships and their single or combined effects on the long-term survival (over 5 years) were statistically analyzed by SPSS15.0.
Results
Loss of p16 was found in 75% of the cases and cyclin D1 overexpression and phosphorylated pRb (p-pRb) were found in 64% and 46%, respectively. Cyclin D1 overexpression was correlated with the p16 loss and pRb inactivation by phosphorylation. The p16 loss was tightly associated with p-pRb. The Kaplan-Meier survival curves disclosed that the cyclin D1-positive group and the p16-negative group showed a rapid decline of survival at the point of about 5 years after surgery and thereafter. The combined actions of cyclin D1 overexpression, loss of p16 and pRb inactivation tended to have an adverse influence on the prolonged survival.
Conclusions
The observation that cyclin D1 overexpression, p16 loss and pRb inactivation were largely found in NSCLCs suggests that they play an important role in pulmonary carcinogenesis. Also, their inverse or positive correlations indicate that the G1/S cell cycle proteins may act alternatively or synergistically on the mechanisms by which tumor cells escape the G1 restriction point. Finally, their solitary or combined actions might have a long-term effect on the survival.
NSCLC is a major type of lung cancer which takes the first place worldwide in the cancer mortality, and much work is now being focused on identifying the disease's pathogenetic mechanisms to get meaningful information for early detection and prevention and to increase the long-term survival. Cell cycle control deregulation is well-known to be a major tumorigenic pathway and this also commonly occurs during pulmonary carcinogenesis (1,2). The aberrant expressions of G1/S cell cycle proteins have been found to play a key role due to their genetic or epigenetic alterations in NSCLCs.
The overexpression of cyclin D1 and the abnormal expression of pRb have been reported in a number of human cancers, including lung cancers (3-6). p16 has also been shown to be one of the most frequently mutated or aberrantly expressed molecules in human cancer, including lung cancer, and this is perhaps equaled or exceeded only by the loss of p53 function (7,8). These facts imply that disruption of the cell-cycle dependent kinase (Cdk)4/6:cyclin D:pRb pathway may be required for the development of most types of human cancer. The studies on the their interactions have shown an inverse relationship between cyclin D1 and pRb alterations, and this supports the hypothesis that alterations of cyclin D1 and pRb are thought to be alternative mechanisms by which tumor cells may escape the G1 restriction point (3). It is also known that there is an inverse correlation between the pRb and p16 expressions and a direct correlation between the pRb and cyclin D1 expressions (4,9). However, most studies on pRb protein have reported the results for the total pRb expression; to the best of our knowledge, no detailed studies have been performed to elucidate the pRb phosphorylation status in NSCLCs. According to a previous study on malignant melanoma (10), pRb is progressively upregulated in advanced and metastatic cases and this increase is paralleled by increased pRb inactivation due to the protein's phosphorylation. Another study also revealed that cyclin D-Cdk4, but not cyclin A/E-Cdk2, phosphorylates Ser780 at the G1 site of pRb during the G1/S transition of the cell cycle (11). The p-pRb at Ser780 did not bind to E2F-1 in vivo, suggesting that this phosphorylation is important for the E2F-1 activation through dissociation of pRb from the pRb-E2F-1 complex (11). Therefore, the evaluation of phosphorylated retinoblastoma protein (p-pRb) using the Phospho-Ser780 Rb antibody (SAT#11197) could become a useful adjunct in clinicopathologic studies.
The reports on survival have usually showed that NSCLC patients with cyclin D1 overexpression and the loss of p16 protein had poor survival (12,13). Yet the results for the total pRb have been controversial (6,9,10) and no data for p-pRb has been accumulated to date. The combined effects of cyclin D1, p16, and pRb proteins on survival have been reported in only a few studies (9,10), and no conclusion has been reached. Therefore, we investigated the relationship of the altered expressions of the main G1/S cell cycle regulators by using cyclin D1, p16 and p-pRb proteins with several prognostic clinicopathologic factors and the long-term survival (over 5 years) in order to establish the key role of cyclin D1 overexpression, p16 loss and pRb phosphorylation in pulmonary carcinogenesis, and we wanted to find their clinical applicability by evaluating their solitary or combined effects on the long-term survival.
A total of 115 consecutive NSCLC patients who underwent complete resection between 1994 and 2002 were selected, with the follow-up period ranging from 15 days to 144 months. The median follow-up period was 43.8 months. The study cohort consisted of patients with stage I-IV NSCLCs. Tumor-node-metastasis (TNM) staging was done according to the revised international system for staging lung cancer (1997). The demographic and clinical data such as age, gender, the smoking history and the TNM status were collected from the hospital records. Death from lung cancer was the terminal event for the survival calculations. Formalin-fixed and paraffin-embedded tumor tissues were stained with hematoxylin-eosin, and the H&E slides were reviewed by a pathologist.
Briefly, the most representative tumor areas and normal areas were carefully selected in pairs and these areas were marked as a 2 mm diameter circle on the H&E slides. The corresponding areas of the donor paraffin blocks were punched by the trephine needle with the hole size being 2 mm. These 2 mm-sized tissue cores were transferred and embedded into the recipient block that had 60 empty 2 mm-sized holes. All the study specimens were sampled at both the tumor and normal regions from each donor block. Multiple 4 (m-thick sections were cut with a microtome and they were transferred to poly-L-lysine-coated slides.
The standard avidin-biotin-peroxidase complex method was used for immunohistochemical examination with monoclonal antibodies against cyclin D1 (SP4, NeoMarkers, CA), p16 (Clone 16P07, Lab Vision, CA), and Rb (Phospho-Ser780, SAT, CA).
The microarrayed tissue sections were deparaffinized with standard xylene and they were hydrated through a series of graded alcohol solutions. The sections were microwaved in 10 mM citrate buffer at 90℃ for 10 min and then they were treated with 3% H2O2-PBS solution to reduce the endogenous peroxidase activity. Then they were incubated with normal bovine serum to reduce the nonspecific antibody binding and they were subsequently subjected to the primary antibody reactions. The primary antibodies for the cyclin D1, p16 and p-pRb proteins were reacted with the sections at room temperature for one hour at the dilution of 1:100, 1:200, and 1:200, respectively. A negative control was incubated without the primary antibodies. Detection of the immunoreactive staining was carried out by the avidin-biotin-peroxidase complex method and using the LSAB kit (DAKO). The sections were subjected to a color reaction with 3,3-diaminobenzidine tetrahydrochloride that contained 3% H2O2 in Tris buffer and they were lightly counterstained with Mayer's hematoxylin.
For evaluation of the expressions of the cyclin D1, p16 and p-pRb proteins, the immunostained cells were considered positive only when distinct nuclear staining was identified, regardless of associated cytoplasmic staining, which was faint and considered non-specific. Cases were considered to show an overexpression for cyclin D1 protein when more than 5% of tumor cells showed definitive nuclear positivity, according to a previous study (9). Cases were considered to have p16 loss when less than 10% of tumor cells were stained with p16 antibody (14). Cases were interpreted as showing pRb inactivation by phosphorylation when more than 10% of the tumor cells showed p-pRb Ser780 protein (10). The cases showing cyclin D1 overexpression and p16 loss were defined as an altered or aberrant expression for these proteins. Those cases with positive immunostaining for p-pRb were considered that the pRb was functionally inactivated by cyclin D/Cdk4-dependent phosphorylation at Serine 780 (10,11).
The comparison of each immunoreactivity between two- or three-categorical clinicopathologic variables and the relationships between cyclin D1, p16, and p-pRb were analyzed by chi-square tests (SPSS 15.0). The survival period was calculated as the time from the date of surgery to the date of death or the last follow-up. The survival data of 115 NSCLC patients was analyzed by the Kaplan-Meier method and then compared by the log rank test. A p value less than 0.05 was defined as statistically significant.
Those data are summarized in Table 1. The study cohort consisted of 87 males and 28 females. The average age for the males and females was 60.6 years and 59.1 years, respectively and that for the combined group was 60.2 years. Of the 115 cases, 89 cases (77%) had a prior history of smoking. Pathologic staging revealed 41 cases of stage I, 30 cases of stage II, 40 cases of stage III and 4 cases of stage IV. The pathological T stages were pT1 in 22 cases, pT2 in 60, pT3 in 27 and pT4 in 6. The pathologic N stages were pN0 in 53 cases, pN1 in 31 and pN2 in 31.
When the tumors were classified by the new WHO criteria of 1999, there were 60 squamous carcinomas (SQC), 38 adenocarcinomas (ADC), 3 adenosquamous carcinomas (ASC), 7 large cell carcinoma (LCC) and 7 pleomorphic carcinomas (PLC). For the purpose of this study, three cases of bronchioloalveolar carcinoma (BAC) were included in the adenocarcinoma category and three ASCs were grouped into the ADCs for the statistical analysis. Of all the histologic types, only 98 cases of SQC and ADC were histologically graded as 12 cases of grade I (well differentiated), 63 cases of grade 2 (moderately differentiated) and 23 cases of grade 3 (poorly differentiated).
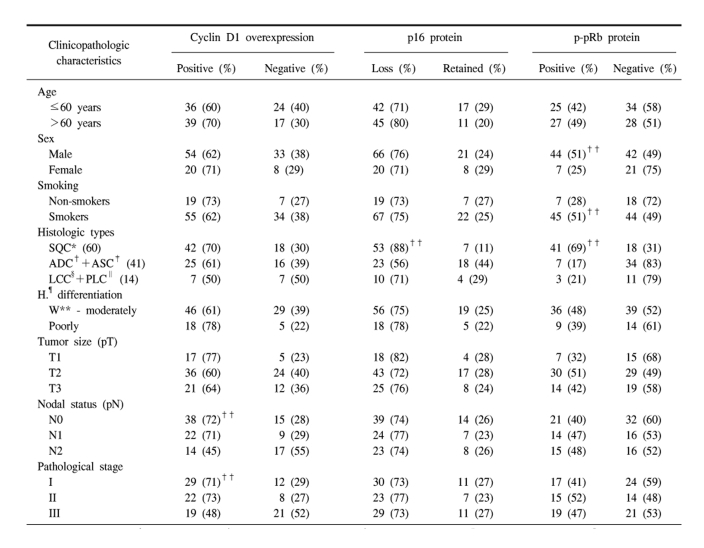
The immunoreactivities for the cyclin D1, p16 and p-pRb proteins in the 115 NSCLCs revealed that cyclin D1 overexpression was found in 74 cases (64%) and p16 loss was found in 86 cases (75%). p-pRb was immunostained in 52 cases (46%). The most frequent alteration of the three cell cycle proteins was the loss of p16 protein (75% of 115 cases), and this was found to be significantly higher in the squamous cell type than in the adenocarcinoma (p=0.001). Cyclin D1 overexpression was significantly associated with the nodal status, which showed the higher rate in the N0-1 than in the N2 stage tumors (p=0.033), and Cyclin D1 overexpression was significantly associated with the pathological stage because the stage I and II tumors revealed cyclin D1 overexpression more frequently than did the stage III tumors (p=0.039). Four cases of stage IV were not included in the statistical analysis for the relationship of an altered expression with the staging because the number of cases was too small to be informative. pRb protein was phosphorylated or functionally inactivated more frequently in males and smokers than in the females and non-smokers, respectively (p=0.017 and p=0.037). The p-pRb protein was also found to be more highly expressed in the squamous cell carcinoma than in the adenocarcinoma or the large cell carcinoma group (p=0.000). Among the clinicopathologic prognostic factors, age, the histologic grades and tumor size were not correlated with any aberrant expression or functional inactivation of the cyclin D1, p16 and p-pRb proteins.
115 microarrayed lung tissues revealed distinct nuclear staining for cyclin D1, p16 and p-pRb proteins in the tumor cells or the normal stromal cells, but the bronchial epithelia showed no evident nuclear immunopositivity for all three proteins. Cyclin D1 was not immunostained in the normal cell components, whereas many NSCLC cases revealed strong nuclear immunoreactivity in the tumor cells (Fig. 1). On the other hand, p16 protein was immunostained in the stromal cell nuclei, but not in many tumor tissues (Fig. 2). p-pRb protein was found to be more highly expressed in the tumor cell nuclei (>10%) than in the activated stromal cell nuclei (<10%) (Fig. 3).
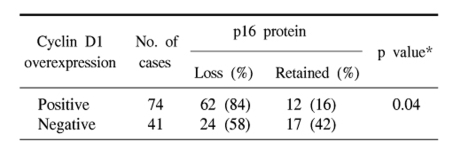
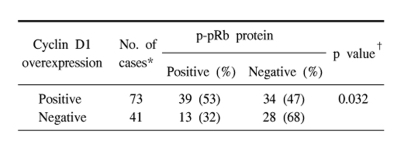
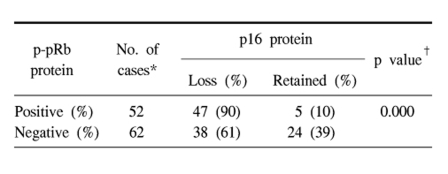
The results of the statistical analysis for the inter-relationship between the cyclin D1 overexpression, p16 loss and pRb phosphorylation are shown in Table 2~4. Overexpression of cyclin D1 was significantly higher in the p16-negative and p-pRb-positive cases. Also, the loss of p16 protein was strongly associated with pRb phosphorylation. To summarize, a cyclin D1 overexpression was inversely correlated with a p16 protein expression, and a cyclin D1 overexpression showed a positive relationship with p-pRb protein. The p16 and p-pRb proteins revealed an inverse relationship between their immunoexpressions.
There was no significant relationship of cyclin D1 overexpression, p16 loss and pRb inactivation with each group's overall survival (p=0.368, p=0.175 and p=0.091, respectively). The patients with a p-pRb expression tended to have shorter survival than the p-pRb(-) cases, but the overall difference was not statistically significant (p=0.091). The combined effect of the altered or inactivated expressions of cyclin D1, p16 and pRb (i.e. cyclin D1/p16, cyclin D1/p-pRb, p16/p-pRb, cyclin D1/p16/p-pRb) showed no significant relationship with each overall survival time for every combination. However, the cases with a cyclin D1 overexpression or a p16 loss tended to show a rapid downhill course from about the 5th year of the survival time, respectively, compared to their counterpart on each Kaplan-Meier survival curve (Fig. 4A, B). Also, the combined altered expressions of the cyclin D1(+)/p16(-), cyclin D1 (+)/p-pRb(+), p16(-)/p-pRb(+) and cyclin D1(+)/p16(-)/p-pRb(-) groups showed the tendency of remarkably reducing the survival rates after about 5 years postoperatively, compared to their counterparts (Fig. 4C~F). Therefore, NSCLC patients with the solitary or mixed components of cyclin D1 overexpression or p16 loss or pRb inactivation by phosphorylation tended to have a consistent adverse effect on the long-term survival after the postoperative 5th year.
Abnormalities in the cell cycle regulation at the G1/S phase appear to be necessary for the development of lung cancers (2-4,6,9,15). Especially, alterations in the Rb pathway of the G1/S cell cycle have been known to occur mostly through inactivation of the cyclin-dependent kinase inhibitor p16INK4 and/or up-regulation of cyclin D1 in non-small cell lung carcinoma (NSCLC) (6,8,11,16). In this analysis, the loss of a p16 expression was the most frequent alteration (75%), followed by a cyclin D1 overexpression (64%) and functional pRb inactivation (46%). Many studies have also reported that NSCLCs preferentially have a loss of the p16 expression, with an inverse relationship with the mutational inactivation of the RB gene or the loss of a pRb expression (8,15,17). The loss of p16 in G1/S cell cycle regulation is known to be pathogenetically linked with a cyclin D1 overexpression, which can cooperate with the loss of p16 in subverting G1 control in the pRb-positive normal cells and tumor cells, but not in pRb-deficient cancers (18). It has also been suggested that the p16 expression should be elevated in pRb-negative tumors and it is normal or suppressed in pRb-positive tumors (19). Aberrations in the p16 and cyclin D1 expressions have been reported to cooperate to deregulate G1 control in multistep carcinognesis, in which the increased expression of cyclin D1 and the decreased expression or loss of p16 would likely provide these cells with a selective growth advantage (5,18). The present study also revealed there is a significant correlation between a cyclin D1 overexpression and the loss of a p16 expression (p=0.004). In this study, cyclin D1 overexpression or p16 loss was significantly associated with p-pRb immunoreactivity (Tables 3, 4). Because the hypothesis of a functional inactivation of pRb due to cyclin- and Cdk-dependent hyperphophoryation was substantiated to a degree, this present data suggests that cyclin D1 overexpression, p16 loss and pRb inactivation could act synergistically or alternatively, as a mechanism by which tumor cells escape the G1 restriction point.
Cyclin D1 overexpression showed a higher rate in this study (64%) than that of other studies that have reported ranges from 42 to 56% in primary NSCLCs (3,8,20). Our immunohistochemical staining showed that the increase in cyclin D1 expression occurred specifically in the tumor cells and not in the stromal or infiltrating inflammatory cells. In this study, cyclin D1 was more significantly overexpressed in the earlier pathological stages (stage I~II) and nodal stages (N0~1) than in stage III and N2, respectively, which suggests that cyclin D1 seems to be involved in initial tumor growth. According to a literature, cyclin D1 overexpression has also been reported to occur as an early event in bronchial neoplasia that precedes the development of squamous cell carcinoma (21-23).
We were unable to detect a p16 expression in normal lung, which is consistent with a previous study that was unable to detect several other cell cycle-related proteins in terminally differentiated lung tissue (8). On our analysis, p16 loss was significantly correlated with the squamous cell type rather thanwith adenocarcinoma (p=0.001). An immunohistochemical study for a p16 protein expression in 171 NSCLCs also showed that the p16 loss was greater in the squamous cell type, and this was correlated with the poorer survival (14). However, we didn't find a significant relationship between a p16 expression and survival in SQC patients.
The loss of pRb function in NSCLC is uncommon compared to that of small cell lung carcinoma (SCLC), but the rate of 6.5 to 60% for the loss of pRb function has been reported in the literatures we reviewed (3,6,24). Most studies on the alteration in pRb protein have used the primary antibody for detecting the phosphorylated and unphosphorylated pRb protein (3,5,6,9), and the results usually show that the lack of total pRb is likely to totally eliminate the checkpoint, making the upstream regulators of the pathway dispensable. Since the p-pRb content seems correlated with the prognosis, evaluation of the p-pRb status has been reported to be a helpful adjunct for clinicopathologic studies on the altered pRb expression (10). The present immunohistochemical analysis on p-pRb revealed that it was found in 46% of the NSCLC patients and it was more frequent in SQC and male smokers than in ADC and female non-smokers. Thus, pRb phosphorylation or inactivation might be considered an important step of carcinogenesis, especially of squamous carcinoma. Thus, pRb inactivation was found to be synergistic with p16 loss in the squamous cell type of NSCLC.
This study showed that 110 (95.6%) of the 115 resectable NSCLCs had at least one among cyclin D1 overexpression, p16 loss and functional pRb inactivation. This result strengthens the emerging key role of the cyclin D-p16-pRb pathway in the control of proliferation and as a regulatory mechanism whose deregulation acts alternatively, and it might represent an obligatory step in the development of human NSCLC.
This study didn't reveal any significant relationship of cyclin D1, p16 or p-pRb proteins with the overall survival of the 115 NSCLC patients, but the survival curves of the patients with a cyclin D1 overexpression and p16 loss tended to show a rapid downhill course at around 5 years of the follow-up period and thereafter. The survival curve for p-pRb revealed the tendency of the p-pRb(+) group to have a lower overall survival rate, but this was not statistically significant (p=0.091) (data not shown). Most reports on the survival analysis for the G1/S cell cycle regulators have generally revealed that NSCLCs with the components of cyclin D1 overexpression, p16 loss and pRb inactivation survived for a significantly shorter period, although there has been some controversial data for altered pRb protein (9-14,25). The previous studies for the survival analysis have usually been done with one component of the G1/S cell cycle regulators, and only a few studies have shown the combined analysis for the relationship between the altered cyclin D1, p16 and pRb expressions and patient survival (9,10). Brambilla et. al showed a shorter survival for the patients with pRb-negative/p16-positive/cyclin D1-negative tumors on their univariate anaylsis (9). In the present study, the cyclin D1(+)/p16(-), cyclin D1(+)/p-pRb(+), p-pRb(+)/p16(-) and cyclin D1(+)/p16(-)/p-pRb(-) groups showed the tendency of remarkably reduced survival rates after about 5 years postoperatively, compared to each of their counterparts. These observations suggest that NSCLC patients with a cyclin D1 overexpression, p16 loss and functional pRb inactivation might have a poor prognosis (or clinical course) after 5 years of follow-up and so they should be observed more carefully.
The observation that the cyclin D1 overexpression, p16 loss and pRb phosphorylation were found in a large number of NSCLCs suggests that the key components of the G1/S cell cycle play an important role in pulmonary carcinogenesis. Also, the inverse or positive correlations between their expressions indicate that the alterations may act alternatively or synergistically on the mechanisms by which tumor cells escape the G1 restriction point. Finally, the solitary or combined effects of cyclin D1 overexpression, p16 loss and pRb inactivation might have a bad prognostic influence on late survival starting at the postoperative 5th year.
References
2. Michalides R. Prognosis of GI cell-cycle regulators: useful for predicting course of disease and for assessment of therapy in cancer. J Pathol. 1999; 188:341–343. PMID: 10440742.
3. Marchetti A, Doglioni C, Barbareschi M, Buttitta F, Pellegrini S, Gaeta P, et al. Cyclin D1 and retinoblastoma susceptibility gene alterations in non-small cell lung cancer. Int J Cancer. 1998; 75:187–192. PMID: 9462706.

4. Zhou JX, Niehans GA, Shar A, Rubins JB, Frizelle SP, Kratzke RA. Mechanisms of G1 checkpoint loss in resected early stage non-small cell lung cancer. Lung Cancer. 2001; 32:27–38. PMID: 11282426.

5. Kang Y, Ozbun LL, Angdisen J, Moody TW, Prentice M, Diwan BA, et al. Altered expression of G1/S regulatory genes occurs early and frequently in transforming growth factor-(1 heterozygous mice. Carcinogenesis. 2002; 23:1217–1227. PMID: 12117781.
6. Burke L, Flieder DB, Guinee DG, Brambilla E, Freedman AN, Bennett WP, et al. Prognostic implications of molecular and immunohistochemical profiles of the Rb and p53 cell cycle regulatory pathways in primary non-small cell lung carcinoma. Clin Cancer Res. 2005; 11:232–234. PMID: 15671551.
7. Kratzke RA, Greatens TM, Rubins JB, Maddaus MA, Niewoehner DE, Niehans GA, et al. Rb and p16INK4a expression in resected non-small cell lung tumors. Cancer Res. 1996; 56:3415–3420. PMID: 8758904.
8. Shapiro GI, Edwards CD, Kobzik L, Godleski J, Richards W, Sugarbaker DJ, et al. Reciprocal Rb inactivation and p16INK4 expression in primary lung cancers and cell lines. Cancer Res. 1995; 55:505–509. PMID: 7834618.
9. Brambilla E, Moro D, Gazzeri S, Brambilla C. Alterations of expression of Rb, p16INK4A and cyclin D1 in non-small cell lung carcinoma and their clinical significance. J Pathol. 1999; 188:351–360. PMID: 10440744.

10. Roesch A, Becker B, Meyer S, Hafner C, Johannes Wild P, Landthaler M, et al. Overexpression and hyperphosphorylation of retinoblastoma protein in the progression of malignant melanoma. Mod Pathol. 2005; 18:565–572. PMID: 15502804.

11. Kitagawa M, Higashi H, Jung HK, Suzuki-Takahashi I, Ikeda M, Tamai K, et al. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996; 15:7060–7069. PMID: 9003781.

12. Epposito V, Baldi A, Tonini G, Vincenzi B, Santini M, Ambrogi V, et al. Analysis of cell cycle regulator proteins in non-small cell lung cancer. J Clin Pathol. 2004; 57:58–63. PMID: 14693837.
13. Mohamed S, Yasufuku K, Hiroshima K, Nakajima T, Yoshida S, Suzuki M, et al. Prognostic implications of cell cycle-related proteins in primary respectable pathologic N2 nonsmall cell lung cancer. Cancer. 2007; 109:2506–2514. PMID: 17487846.
14. Huang CI, Taki T, Higashiyama M, Kohno N, Miyake M. p16 protein expression is associated with a poor prognosis in squamous cell carcinoma of the lung. Br J Cancer. 2000; 82:374–380. PMID: 10646891.

15. Kaye FJ. RB and cyclin dependent kinase pathways: defining a distinction between Rb and p16 loss in lung cancer. Oncogene. 2001; 21:6908–6914. PMID: 12362273.

16. Betticher DC, White GRM, Volanthen S, Liu X, Kappeler A, Altermatt HJ, et al. G1 control gene status is frequently altered in resectable non-small cell lung cancer. Int J Cancer. 1997; 74:556–562. PMID: 9355981.
17. Sakaguchi M, Fujii Y, Hirabayashi H, Yoon HE, Komoto Y, Que T, et al. Inversely correlated expression of p16 and Rb protein in non-small cell lung cancers: an immunohistocxhemical study. Int J Cancer. 1996; 65:442–445. PMID: 8621224.
18. Lukas J, Aagaard L, Strauss M, Bartek J. Oncogenic aberrations of p16INK4/CDKN2 and cyclin D1 cooperate to deregulate G1 control. Cancer Res. 1995; 55:4818–4823. PMID: 7585513.
19. Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cylce control causing specific inhibition of cyclin D/CDK4. Nature (Lond.). 1994; 366:704–707. PMID: 8259215.
20. Betticher DC, Heighway J, Hasleton PS, Altermatt HJ, Ryder WDJ, Cenry T, et al. Prognostic significance of CCND1(cyclin D1) overexpression in primary resected non-small cell lung cancer. Br J Cancer. 1996; 73:294–300. PMID: 8562333.
21. Schauer E, Siriwardana S, Langan TA, Sclafani RA. Cyclin D1 overexpression vs. retinoblastoma inactivation: Implications for growth control evasion in non-small cell and small cell lung cancer. Proc Natl Acad Sci USA. 1994; 91:7827–7831. PMID: 8052667.

22. Betticher DC, Heighway J, Thatcher N, Hasleton PS. Abnormal expression of CCND1 and RB1 in resection margin epithelia of lung cancer patients. Br J Cancer. 1997; 75:1761–1768. PMID: 9192978.
23. Lonardo F, Rusch V, Langenfeld J, Dmitrovsky E, Klimstra DS. Overexpression of cyclin D1 and E is frequent in bronchial preneoplasia and precedes squamous cell carcinoma development. Cancer Res. 1999; 59:2470–2476. PMID: 10344760.
24. Gregorc V, Darwish S, Ludovini V, Pistola L, De Angelis V, Mihaylova Z, et al. The clinical relevance of Bcl-2, Rb and p53 expression in advanced non-small cell lung cancer. Lung Cancer. 2003; 42:275–281. PMID: 14644514.

25. Singhai S, Vachani A, Antin-Ozerkis D, Kaiser LR, Albelda SM. Prognostic implications of cell cycle, apoptosis, and angiogenesis biomarkers in non-small cell lung cancer: a review. Clin Cancer Res. 2005; 11:3974–3986. PMID: 15930332.

Fig. 1
Cylin D1 overexpression in squamous cell carcinoma is characterized by the distinct nuclear immunoreactivity in most of the tumor cells, which is in contrast to the lack of immunostaining on the adjacent stromal cells (×200).
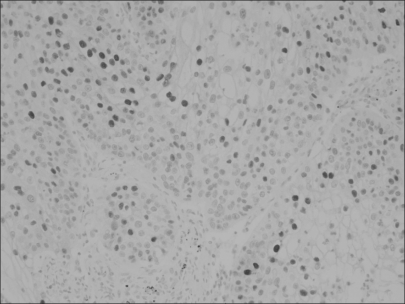
Fig. 2
Loss of p16, a cell cycle inhibitor protein, is observed in a few non-small cell carcinoma nests, which is contrast with the evident immunopositivity in the normal fibroblastic stromal cell nuclei (×200).

Fig. 3
Phosphorylated pRb protein is immunostained in a large proportion of the squamous carcinoma cell nuclei, which is also noted but less frequently in the adjacent activated stromal cell nuclei (×400).
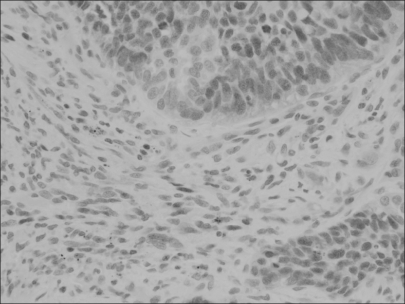
Fig. 4
The Kaplan-Meier survival curves of the 115 non-small cell lung carcinoma patients, according to the solitary or combined immunoexpressions of cyclin D1, p16 and pRb proteins. (A) The cumulative survivals of the cyclin D1-positive and -negative cases were not significantly different during the overall follow-up period (p=0.368), but there was a tendency of the cyclin D1-positive cases to have markedly reduced survival rates after about 5 years (as indicated by a vertical line). (B) The p16-negative cases tended to survive for a shorter time than the p16-positive cases, not in the overall follow-up period, but after about 5 years of follow-up. (C~F) The cumulative survivals of the cyclin D1+/p16-, cyclin D1 +/p-pRb+, p16-/p-pRb+, and cyclin D1+/p16-/p-pRb- groups also revealed the tendency to have markedly reduced survival rates, especially after the postoperative 5th year.
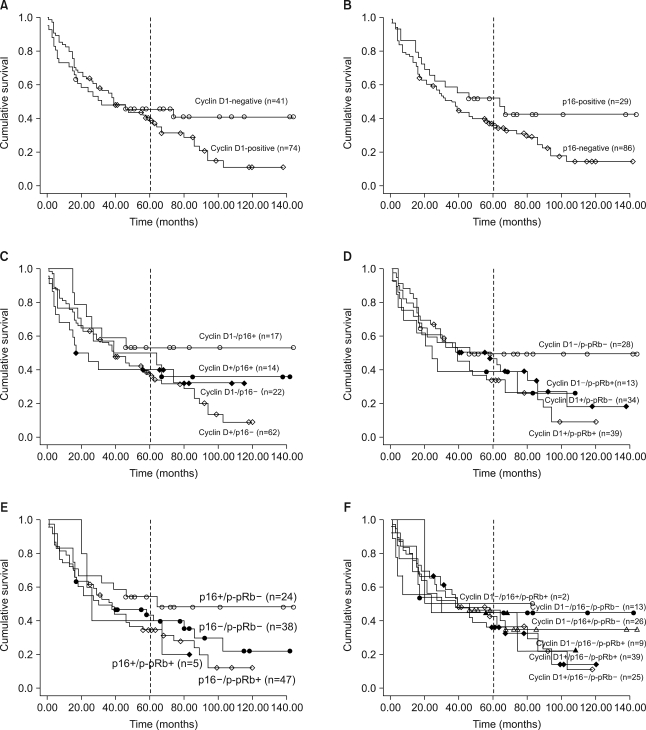
Table 1
Correlation between clinicopathologic parameters and immunoexpressions of G1/S cell cycle markers such as cyclin D1, p16, and phosphorylated pRb (p-pRb)




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download