Abstract
Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome (TTP-HUS) is a rare condition that is severe and may be fatal. Adverse reactions to drugs increasingly are reported as probable causes of TTP-HUS. Many chemotherapeutic agents have also been implicated in causing TTP-HUS. We reported a woman with metastatic renal cell carcinoma who presented with TTP-HUS associated with sunitinib. She had gross hematuria and generalized edema. The hemoglobin concentration was 8.9 g/dl and the platelet count was 46,000/mm3. Her reticulocyte count was increased to 4.1% and the peripheral blood smear revealed red blood cell fragmentation and spherocytes. The patient completely recovered after discontinuing the use of sunitinib and undergoing plasmapheresis. Because of the increasing use of sunitinib in the treatment of cancer patients, oncologists should be aware of the possibility of TTP-HUS related to sunitinib, as early recognition and prompt therapeutic intervention can be beneficial.
Thrombotic thrombocytopenic purpura-hemolytic uremic synrome (TTP-HUS) is a rare condition that is severe and may be fatal. TTP-HUS is a spectrum of syndromes with common features of thrombocytopenia and microangiopathic hemolytic anemia (MAHA). Although the majority of cases have no evident triggering event, several etiologic causes have been identified. Adverse reactions to drugs are increasingly reported as probable causes of TTP-HUS. Recognition of a drug-associated etiology in patients with TTP-HUS is critical to avoid re-exposure and recurrent events (1,2).
Chemotherapeutic agents have also been implicated in causing TTP-HUS, such as mitomycin C, cisplatin, bleomycin, doxorubicin, 5-fluorouracil, interferon-alfa, carboplatin, daunorubicin, dacarbazine, oxaliplatin, lomustine, vinblastine, tamoxifen, carmustine, tretinoin, cytarabine, and gemcitabine (3).
In this report, we describe a case of TTP-HUS following the use of sunitinib in a patient with renal cell carcinoma (RCC).
A 62-year-old woman was admitted to the hospital with gross hematuria and generalized edema. The symptoms had appeared one week earlier and had gradually worsened. She also complained of anorexia, nausea, and dyspepsia. She reported no fatigue, asthenia, or diarrhea. The patient had a history of left RCC, which was treated with a left radical nephrectomy in 1991. She developed pulmonary metastases four and 10 years after the nephrectomy, and was treated with wedge resections. She received adjuvant interferon therapy after the second wedge resection of her lung. Four months prior to her current admission, she developed lung and iliac bone metastases with invasion into the iliopsoas muscle and received palliative radiation therapy to the right pelvis. Tumor responses to radiation were stable disease. Three weeks prior to her current admission, sunitinib was initiated as a single agent. The drug was given orally daily (50 mg) on a four-week-on and two-week-off schedule.
The patient had a medical history of proteinuria of unknown origin that had been controlled with valsartan for the past four years. She also took a lipid-lowering agent. She had no history of hypertension. On examination, the patient was alert and appeared chronically ill. Her temperature was 37.0℃; blood pressure, 160/109 mm Hg; pulse rate, 90 beats per minute; and respiratory rate, 18 breaths per minute. There were petechiae and pitting edema (2+) in the legs bilaterally, and the right pelvic mass was palpable. The remainder of her physical examination was normal. A neurologic examination did not reveal any abnormalities. There was no rash, hand-foot syndrome or skin discoloration.
The white blood cell count was 4,310/mm3, the hemoglobin concentration was 8.9 g/dl, and the platelet count was 46,000/mm3. Her reticulocyte count was increased to 4.1% and the peripheral blood smear revealed red blood cell fragmentation and spherocytes. The lactic dehydrogenase (LDH) was 1,299 IU/L (reference range, 240~480 IU/L), the haptoglobin was 2.0 mg/dl (reference range, 30~200 mg/dl), and the plasma hemoglobin was 93.8 mg/dl (<5 mg/dl). The total protein was 6.1 g/dl, the albumin was 2.9 g/dl, the blood urea nitrogen was 16.1 mg/dl, and the serum creatinine was 1.64 mg/dl. The serum sodium, potassium, phosphate, calcium and uric acid levels were normal. The prothrombin and partial-thromboplastin times, as well as the bilirubin and aminotransferase levels also were normal. The Coombs direct and indirect tests were negative. A urinalysis showed severe proteinuria (10.6 g/day) compared with the level two months earlier (0.55 g/day). The ADAMTS13 level was not studied.
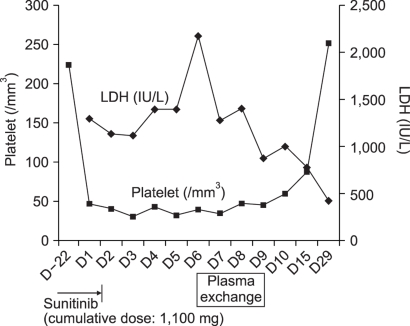
Objective laboratory results support our diagnosis of TTP-HUS in our patient. While undergoing treatment with interferon alfa or radiation in the past, our patient showed no clinical or biological signs of thrombotic microangiopathy. We considered alternative explanations for this patient's TTP-HUS, but did not find any other likely causal relationship. There were no other new medications, except sunitinib, and no clinical symptoms suggested the source of infection. The diagnosis of TTP-HUS was made, sunitinib was stopped. The management of the hypertension was initiated with amlodipine and furosemide. On the seventh day after hospital admission, plasmaphreisis was started. After two sessions of plasmapheresis, her symptoms were markedly improved and blood pressure was normalized. Schistocytes disappeared in the peripheral blood smear. Her platelet count and LDH were normalized by the follow-up visit to the clinic three weeks after discharge (Fig. 1).
Sunitinib (Sutent; Pfizer, NY) is an oral, multi-targeted tyrosine kinase inhibitor with anti-tumor and anti-angiogenic activities. Sunitinib has been identified as a potent inhibitor of vascular endothelial growth factor receptors (VEGFR)-1, -2, and -3, platelet derived growth factor receptors (PDGFR)-a and -b, stem cell factor receptor (KIT), Fms-like tyrosine kinase-3 (FLT3), colony stimulating factor receptor Type 1 (CSF-1R), and glial cell-line-derived neurotrophic factor receptor (RET). Sunitinib is approved for treatment of advanced RCC and gastrointestinal stromal tumors (GIST) after disease progression on, or intolerance to, imatinib mesylate. This drug has also been shown to have antitumor activity in phase I and II trials in patients with advanced malignancies, including breast cancer, neuroendocrine tumors, colorectal cancer, sarcomas, thyroid cancer, melanomas, and non-small cell lung cancer (4,5).
Sunitinib is generally well-tolerated. Adverse events reported in patients with RCC who received sunitinib were mostly of mild or moderate severity. Frequent adverse reactions were fatigue, asthenia, diarrhea, nausea, mucositis/stomatitis, vomiting, dyspepsia, abdominal pain, constipation, hypertension, rash, hand-foot syndrome, skin discoloration, altered taste, anorexia, and mild bleeding. Hypothyroidism and cardiotoxicity are also known as important sunitinib-related adverse events (6,7).
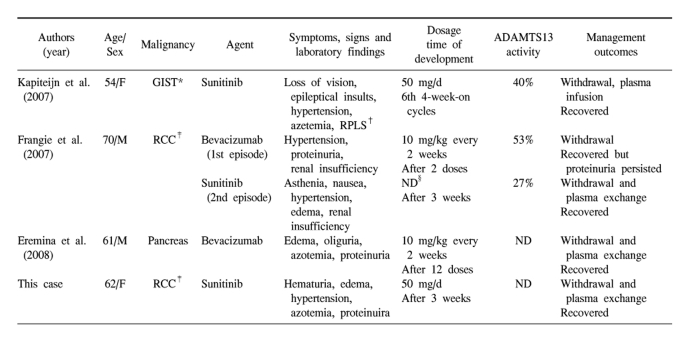
TTP-HUS due to sunitinib has been previously reported only twice in the literature (8,9). Clinical features of the previous reported TTP-HUS associated with anti-VEGF agents are summarized in Table 1 (8-10). The common characteristics of these cases are as follows. First, all patients presented with renal complications. Also, except for one case that has no description of blood pressure, all patients developed the onset or exacerbation of pre-existing hypertension. But neurologic abnormalities showed in one patient, probably thought to be due to coexisting reversible posterior leukoencephalopathy syndrome (RPLS). Second, they do not seem to be dose-dependent. In contrast, the patients using mitomycin C seldom develop TTP-HUS until they have received a cumulative dose of greater than 30 mg/m2 and as the cumulative dose rises, so does the risk of this complication (11). Similarly, the risk of gemcitabine-associated TTP-HUS seems to increase when the cumulative dose approaches 20,000 mg/m2 (3). Third, the prognosis of TTP-HUS associated with anti-VEGF agents was very good. In general, the prognosis of patients with chemotherapy-induced TTP-HUS remains poor. Plasma exchange is ineffective in most patients (11). But in TTP-HUS associated with anti-VEGF agents, the signs of TTP-HUS resolved after discontinuing the use of the drug and undergoing plasma exchange or infusion. There was persistence of proteinuria in one case.
Significant advances have been made in understanding the pathogenesis of TTP-HUS since the discovery of ADAMTS-13, the enzyme that regulates the size of the von Willebrand factor (VWF) multimers. The idiopathic TTP-HUS is mainly caused by a severe ADAMTS-13 deficiency, yet relationships between VWF-cleaving metalloproteinase and secondary TTP-HUS including drug-associated cases are still unclear. Severe deficiency of ADAMTS13 (<5%) has not been found in patients with TTP-HUS associated with anti-VEGF agents. But, recent studies have suggested that a mild/moderate deficiency in circulating ADAMTS-13 activity levels may also be involved in the pathogenesis secondary TTP (12). As testing for ADAMTS13 becomes more readily available, the strength of this association should become clearer.
The mechanism by which anti-VEGF agents cause thrombotic microangiopathy is unclear, but one recent study provided direct experimental evidence of the mechanism (10). Eremina et al. showed that the production of VEGF by podocytes is indispensable for maintenance of the adjacent glomerular endothelium in the murine model. Interruption of VEGF function results in renal damage, which suggests that thrombotic microangiopathy in patients who received bevacizumab results from a reduction in glomerular VEGF. This association does not exclude the possible contribution of other pathways, such as PDGFR.
Although the advent of novel molecular targeted agents represent a substantial advance in the treatment of disseminated cancer, their spectrum of adverse effects may be broader than predicted. Active post-marketing surveillance of these drugs is essential to ensure that rare, but serious toxicities, such as TTP-HUS are recognized. Our case shows that sunitinib can cause TTP-HUS. Because of the increasing use of sunitinib in the treatment of cancer patients, oncologists should be aware of the possibility of TTP-HUS related to sunitinib, as early recognition and prompt therapeutic intervention can be beneficial.
References
1. Medina PJ, Sipols JM, George JN. Drug-associated thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Curr Opin Hematol. 2001; 8:286–293. PMID: 11604563.

2. Zakarija A, Bennett C. Drug-induced thrombotic microangiopathy. Semin Thromb Hemost. 2005; 31:681–690. PMID: 16388419.

3. Zupancic M, Shah PC, Shah-Khan F. Gemcitabine-associated thrombotic thrombocytopenic purpura. Lancet Oncol. 2007; 8:634–641. PMID: 17613425.

4. Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007; 25:884–896. PMID: 17327610.

5. Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, et al. Food and Drug Administration drug approval summary: Sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007; 12:107–113. PMID: 17227905.

6. Rini BI, Tamaskar I, Shaheen P, Salas R, Garcia J, Wood L, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007; 99:81–83. PMID: 17202116.

7. Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007; 370:2011–2019. PMID: 18083403.

8. Kapiteijn E, Brand A, Kroep J, Gelderblom H. Sunitinib induced hypertension, thrombotic microangiopathy and reversible posterior leukencephalopathy syndrome. Ann Oncol. 2007; 18:1745–1747. PMID: 17890216.

9. Frangie C, Lefaucheur C, Medioni J, Jacquot C, Hill GS, Nochy D. Renal thrombotic microangiopathy caused by anti-VEGF-antibody treatment for metastatic renal-cell carcinoma. Lancet Oncol. 2007; 8:177–178. PMID: 17267332.
10. Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008; 358:1129–1136. PMID: 18337603.

11. Dlott JS, Danielson CF, Blue-Hnidy DE, McCarthy LJ. Drug-induced thrombotic thrombocytopenic purpura/hemolytic uremic syndrome: a concise review. Ther Apher Dial. 2004; 8:102–111. PMID: 15255125.

12. Franchini M, Montagnana M, Targher G, Lippi G. Reduced von Willebrand factor-cleaving protease levels in secondary thrombotic microangiopathies and other diseases. Semin Thromb Hemost. 2007; 33:787–797. PMID: 18175284.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download