INTRODUCTION
The transforming growth factor β (TGF-β) family is a multifunctional group of cytokines that includes three mammalian TGF-βs, activins, inhibins, and a number of bone morphogenetic proteins (BMPs), all of which can regulate processes such as cell growth, terminal differentiation, apoptosis, immune responses, and expression of extracellular matrix through transcriptional regulation of a diverse number of gene targets (
1-
3).
The TGF-β superfamily members signal through a family of transmembrane receptors that have intrinsic cytoplasmic serine/threonine kinase activity. The binding of a ligand to a type II receptor (TGF-βRII) results in the recruitment and transphophorylation of type I receptors (TGF-βRI), which then signal downstream responses and induce carboxy-terminal serine phosphorylation of a set of cytoplasmic signal-transducing proteins collectively referred to as Smad proteins (
1-
4). Receptor-activated Smads then interact transiently with specific, ligand-activated TGF-βRI and are phosphorylated. Pathway-specific Smads include Smads 1, 5, and 8, which mediate BMP signaling, and Smads 2 and 3, which mediate TGF-β and activin signaling. A phosphorylated pathway-specific Smad heterodimerizes with Smad4, and this complex then translocates to the nucleus to transactivate specific target genes. Smad4 is functionally unique among the Smads and is not regulated by phosphorylation, but instead acts as a common mediator of all of the pathway-specific Smads and as a common mediator of TGF-β, activin, and BMP signaling responses. Inhibitory Smads disrupt signal transduction by preventing phosphorylation of pathway-specific Smads. Smad6 appears to inhibit BMP signaling, while Smad7 is more involved in the inhibition of TGF-β-dependent signaling. In contrast to the pathway-specific Smads, the inhibitory Smads are primarily localized to the nucleus in the absence of a ligand. However, they accumulate in the cytoplasm upon receptor activation (
1-
4).
TGF-β is a potent growth inhibitor of most cells (
5). Cellular insensitivity to growth inhibition by TGF-β is a hallmark in the genesis and progression of human cancer, which can be directly linked to inactivating mutations or the loss of expression of various signaling molecules whose activities are regulated by TGF-β. TGF-β and its intracellular signaling proteins are widely established tumor suppressors (
6), and mutation or loss of the gene responsible for TGF-β receptor expression has been noted in cases of cutaneous and noncutaneous T-cell lymphoma, B-cell lymphoma, and Hodgkin's lymphoma (
5,
7,
8). Therefore, loss of TGF-β receptors nullifies the immunosuppressive properties of TGF-β in lymphocytes and enhances cell proliferation, indicating that this may be an important step in the development of malignant lymphoma.
The Smads for TGF-β also appear to function as tumor suppressors. For instance, mutations that inactivate Smad2 have been identified in human colorectal and lung cancers, whereas mutations that lead to the inactivation or loss of Smad4 expression have been found in human pancreatic, breast, colorectal, lung, ovarian, and head and neck cancers (
9). Moreover, the Smad3 gene functions as a tumor suppressor in mice. Therefore, targeted disruption of Smad3 leads to the formation of colorectal adenocarcinomas that are capable of penetrating the intestinal wall and metastasizing to distant locations. This finding suggests that the gene encoding Smad3 is a tumor suppressor, similar to the genes that encode for Smad2 and Smad4 (
10). In spite of the information available regarding Smad proteins, little is known about their expression pattern during lymphomagenesis. Therefore, in this study, the localizations of Smad3 and Smad4 in T-cell lymphoma tissues were compared in order to elucidate their roles in lymphomagenesis.
Go to :

MATERIALS AND METHODS
Paraffin-embedded tissue blocks of surgically resected tissues, diagnosed as T-cell lymphomas based on the new WHO classification, were retrieved from the surgical files. Immunohistochemical detection was used for B-cell (CD20), T-cell (CD3), and NK-cell (CD56) markers in paraffin-embedded sections in order to confirm the T- or NK-cell nature of these tumors. Twenty-six cases of T-cell lymphoma broke down as follows: 4 cases of acute lymphoblastic lymphoma, 8 cases of peripheral T-cell lymphoma-unspecified, 10 cases of nasal or nasal type NK/T-cell lymphoma, 2 cases of anaplastic large cell lymphoma, 1 case of angioimmunoblastic lymphoma, and 1 case of adult T-cell lymphoma. All cases were included in this study.
1) Immunohistochemistry
Immunohistochemical analysis was performed on formalin-fixed, paraffin-embedded materials using a primary antibody for Smad3 (diluted 1:50) (Zymed Laboratories, San Francisco, CA (51~1,500)) or Smad4 (diluted 1:50) (Santa Cruz Biotechnology, Santa Cruz, CA, (B-8)). Briefly, the sections were deparaffinized in xylene, rehydrated, washed in distilled water, immersed in 10 mM citrate buffer (pH 6), and then microwaved for 10 minutes. Next, the sections were treated with a 3% H2O2 solution to reduce endogenous peroxidase activity, and then washed in phosphate buffer saline. The samples were then incubated with primary antibodies. Detection of immunoreactive staining was achieved by the streptavidin biotin method using an LSAB kit (DAKO, Carpinteria, CA). Sections were subjected to a color reaction with diaminobenzidine, and then counterstained with Mayer's hematoxylin. Tumors were considered to be positive only when a distinct nuclear staining comparable to that of normal lymphoid cells (tonsil and thymus) could be demonstrated.
2) Statistical analysis
The differences in Smad4 positivity in precursor and peripheral T-cell lymphomas were assessed using Fisher's exact test.
Go to :

RESULTS
Normal squamous epithelium is positive for Smad3 and Smad4; therefore, it was used as the positive control. More than 50% of the thymocytes in the thymus showed strong nuclear staining for Smad3 (
Fig. 1A), and nearly all of the cells in the germinal centers and more than 50% of the paracortical cells in the tonsils were positive for Smad3, with moderate staining intensity (
Fig. 1B). When Smad4 immunostaining was conducted, nearly all of the thymocytes showed strong to moderate nuclear staining in the thymus (
Fig. 1C), and nearly all of the cells in the germinal centers showed diffuse cytoplasmic staining, with most of them exhibiting nuclear positivity, as well. In addition, more than 50% of the cells in the paracortex of the tonsil were positive for Smad4, with moderate staining intensity (
Fig. 1D).
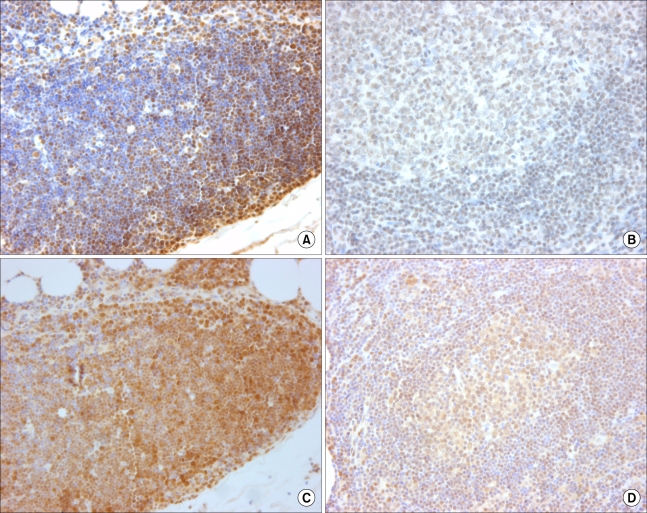 | Fig. 1When Smad3 immunostaining was conducted, more than 50% of the thymocytes showed strong nuclear staining (A), and more than 50% of the paracortical cells were positive with moderate staining intensity (B). When Smad4 immunostaining was conducted, nearly all of the thymocytes showed strong to moderate nuclear staining (C), and more than 50% of the cells in the paracortex were positive, with moderate staining intensity (D). 
|
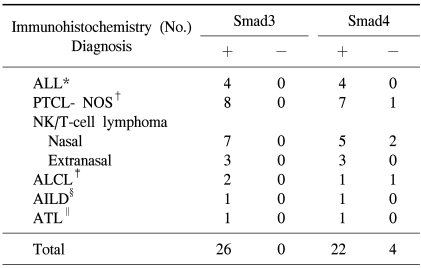
When the malignant lymphomas were evaluated, all of the cases were strongly positive for Smad3 (
Fig. 2). However, 22 cases were also moderately to strongly positive for Smad4 (
Fig. 3), and 4 cases (15%) were negative for Smad4 (
Table 1). When Smad3 was evaluated, all of the precursor lymphomas were positive, and nearly all of the tumor cells within the lymphomas were positive with a staining intensity (strong) similar to that seen in the non-neoplastic thymocytes. Furthermore, analysis of all of the peripheral T-cell lymphomas revealed that most of the tumor cells were positive and had a stronger staining intensity than the paracortical mature T-cells did. When Smad4 was evaluated, all acute lymphoblastic lymphomas showed nuclear positivity. In contrast, one unspecified peripheral lymphoma, two nasal NK/T cell lymphomas, and one anaplastic large cell lymphoma were negative for Smad4, although these differences were clinically insignificant (p=0.489). Staining intensities similar to those observed in non-neoplastic thymocytes were observed in nearly all tumor cells in precursor T-cell lymphomas, and positive peripheral T-cell lymphomas exhibited more intense staining than that observed in non-neoplastic lymphoid tissue.
 | Fig. 2When the T-cell lymphomas were evaluated, all of the cases were strongly positive for Smad3. 
|
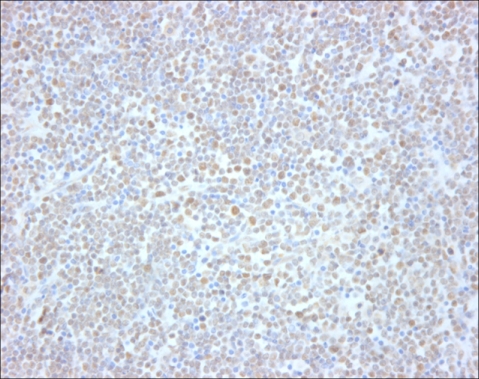 | Fig. 3Twenty-one T-cell lymphomas were moderately to strongly positive for Smad4, and four cases (15%) were negative for Smad4. 
|
Table 1
Expression of Smad3 and Smad4 in T-cell lymphomas

Go to :

DISCUSSION
TGF-β and BMP are widely expressed during embryonic development, especially in tissues in which the inductive factors produced at the mesenchymal-epithelial interfaces influence cell differentiation and organogenesis (
2). The early discovery of the anti-proliferative effects of TGF-β on T-cells in vitro suggested that TGF-β plays a crucial role in T-cell regulation (
11), and it has been shown that TGF-β1 plays a major role in T-cell differentiation and function. In addition, TGF-β1 mediates inhibition of the proliferation of freshly isolated human thymocytes in response to IL-2, IL-4, and IL-7 (
12). Thymocytes and thymic epithelial cells express TGF-β, which limits the developmental progression of thymocytes (
13), and TGF-β type I receptor and nuclear translocated phosphorylated-Smad2, a hallmark of active TGF-β/activin signaling, have been detected in the thymus (
13). These findings are similar to those seen in the present study, in which more than 50% of the thymocytes showed strong nuclear staining for Smad3, and nearly all of the thymocytes showed strong to moderate nuclear staining for Smad4.
In normal lymph nodes, TGF-β is produced by follicular dendritic cells within the microenvironment of the secondary lymphatic follicles (
14). However, in the reactive lymphoid tissues, less than 5% of the small lymphocytes have been shown to be positive for TGF-β (
15), and most of the small lymphocytes, granulocytes, and germinal centers are non-reactive (
16,
17). In a previous experiment, a mouse model was used to demonstrate that all of the tissues that were examined expressed at least one of the BMP-specific Smads (1 or 5) and one of the TGF-β/activin-specific Smads (2 or 3). However, the amount of nuclear staining for these Smad proteins varied depending upon the tissue and cell type (
2). In lymphoid tissue, the stromal cells and T cells of the developing thymus showed widespread expression of the common mediator, Smad4, and moderate expression of the TGF-β-specific Smads, 2 and 3, with Smad3 being found primarily in the nucleus. In the spleen, Smads 2 and 4 are the most highly expressed Smads (
2). However, little information has been gathered regarding the expression patterns of Smad proteins in human lymphoid tissue. In this study, nearly all of the cells in the germinal centers and more than 50% of the paracortical cells were positive for Smad3. In addition, most of the cells in the germinal centers and the paracortical regions showed nuclear positivity for Smad4. TGF-β plays an important role in regulating the balance between proliferation and differentiation of hematopoietic cells (
18,
19), and it is also an important regulator of immune cell development and function (
20). The generalized expression patterns of Smad3 and Smad4 found in lymphoid tissues, including thymus and tonsil, suggest that TGF-β-specific Smads may be actively involved in signal transduction in the TGF-β signaling pathway, and that they function to maintain the homeostasis of lymphoid cells in human lymphoid organs.
In a study conducted on non-Hodgkin's lymphomas, only a few small lymphocytes were positive for TGF-β, and no TGF-β was detected in tumor cells obtained from follicular lymphomas or peripheral T-cell lymphomas (
15). Conversely, the same study found that 30% of the Reed-Sternberg cells were positive for TGF-β, and a large number of medium-sized T-lymphocytes were also positive for TGF-β in the Hodgkin's lymphoma samples (
15). In addition, another study found that all of the low- and high-grade gastric B-cell lymphomas were negative or weakly positive for TGF-β1 (
13). Another study involving diffuse large B-cell lymphomas found that the tumors were all positive for Smad3, but that some of these cases (19%) were negative for Smad4 (
21).
To date, there has been little data generated regarding the expression patterns of Smads in T-cell lymphoma. In our study, all T- or NK-cell lymphomas were strongly positive for Smad3. In contrast, one group reported decreased expression of Smad3 by Western blotting in T-cell leukemia (
22), suggesting that the level of Smad3 protein expressed by T-cells might be an important determinant in the suppression of T-cell tumorigenesis. Generally, the level of Smad3 protein in T-cells is known to be directly correlated with the gene dosage: levels in Smad +/- mice are approximately half those seen in Smad3 +/+ mice, and the loss of Smad3 alone is insufficient to initiate T-cell tumorigenesis. In their study, 10% of Smad3 +/-, p27 -/- mice developed T-cell lymphoblastic leukemia. Smad3 was expressed from the retained allele, as evidenced by immunohistochemistry detection in solid tumors and Western blotting detection in lysates of purified lymphoblasts (
22). Therefore, our cases showing Smad3 protein expression might be Smad3 +/- associated with alterations in other factors that control the activation, proliferation, and viability of T-cells such as p27.
However, some of T or NK-cell lymphomas in our study (4/26) were negative for Smad4. All acute lymphoblastic lymphomas were positive, in contrast to four peripheral T-cell lymphomas showing Smad4 negativity. Genetic changes, including DNA mutations of the Smad genes, have been rarely reported in hematopoietic tumors (
18). Therefore, loss of Smad4 expression might be related to an epigenetic mechanism or might be induced by alterations at the post-DNA level.
Our findings suggest that the TGF-β signaling pathway, mediated through the Smad proteins, generally operates in this type of lymphoma because the nuclear localization of Smad proteins pinpoints the area in which signaling is particularly active (
2).
High expression of inhibitory Smads may ameliorate or attenuate strong signaling by receptor-activated Smads, and the expression of other factors, such as BAMBI/Eyb/nma, Ski, SnoN, and SARA, may modulate this signaling. Moreover, it has been shown that signaling by members of the TGF-β superfamily is not exclusively Smad-dependent (
2). TGF-β-induced growth stimulation occurs in a Smad-independent manner and is accompanied with increased Erk phosphorylation and elevated cyclin D1 expression (
23). However, further studies to evaluate these factors in normal lymphoid tissues and lymphoma tissues are needed to evaluate the reason for Smad3 and Smad4 expression in the development of lymphoid organs and in T-cell lymphomagenesis.
Disturbance of the lymphocyte TGF-β signaling pathway enhances cell proliferation and may contribute to the development of malignant lymphoma. In this study, a small portion of the lymphomas exhibited loss of Smad4 expression. Smads can function as tumor suppressors to ameliorate TGF-β signaling in lymphoid tissues. Further studies using larger samples will be required to correlate the loss of Smad4 expression with other clinicopathologic parameters, as well as to determine its clinical significance in the setting of T-cell lymphoma.
Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download