Abstract
Purpose
Anthracycline can effectively treat hematologic malignancies, but has significant risk of cardiotoxicity. We measured the clinical correlation between brain natriuretic peptide (BNP) and anthracycline-induced cardiotoxicity.
Materials and Methods
Between March 2005 and March 2007, 86 patients with acute leukemia, malignant lymphoma, or multiple myeloma receiving systemic chemotherapy with anthracycline were enrolled in the Department of Hemato-oncology, Kosin University Gospel Hospital. We investigated the relationship between BNP level and cardiotoxicity through echocardiography, electrocardiography, BNP levels, and symptoms of heart failure at each chemotherapy cycle.
Results
Of the 86 participants (mean age, 48.5 years; range 20~65 years), cardiotoxicity developed in 21 patients (24.4%), with 2 patients showing arrhythmia only, 17 patients with transient aspects of heart failure, and 2 patients with chronic heart failure. Cardiotoxicity related to serum BNP level, age, cumulative dose of anthracycline, accompanying chronic disease, and elevated level of troponin-I. Heart failure was more common if BNP levels reached 100 pg/ml at least once.
Anthracyclin chemotherapeutic agents such as Doxorubicin (adriamycin) and epirubicin show strong activity to kill malignant cells, and thus they have been used for various kinds of malignant diseases. Nevertheless, cardiotoxicity induced by anthracyclin may cause long term side effects after treatment, hence, it has been considered to be an important problem (1,2). In other words, in cases of malilignant lymphoma, acute leukemia, multiple myeloma and other hematological tumors or breast cancer among solid tumors that could be completely cured or long term survival is possible by the treatment with chemotherapy including anthracyclin, but heart failure developed during treatment or after treatment is a severe side effect that threatens the life of patients and deteriorates quality of life (3-8).
Cardiotoxicity induced by anthracyclin is associated with the cumulative dose of drug, and in addition, it has been reported that in cases accompanying other risk factors such as age, female and others, the possibility of developing cardiotoxicity is high (9,12,13). Thanks to the development of diverse chemotherapeutic agents and the improvement of adjuvant therapies, malignant diseases including numerous hematological diseases could be completely cured (7).
Therefore, to predict and to prevent the long term toxicity caused by chemotherapeutic agents accompanied after treatment are important, and strategies for them are considered to be required. For the diagnosis of cardiotoxicity including heart failure, methods using ultrasonography has been used widely presently, and it is useful for the diagnosis of cases developed complications, nonetheless, it does not show satisfactory results for early detection (10). Brain natriuretic peptide (BNP) is a type of cardiac neurohormone secreted by the ventricles due to the increase of ventricular volume and pressure, elevation of serum concentration of BNP has been known to be significantly correlated to the left ventricular end diastolic pressure as well as pulmonary capillary wedge pressure in heart failure patients (14). Pertinent to this, since serum concentration of BNP reflects left ventricular dysfunction, it has been applied to the diagnosis as well as treatment of heart failure patients and to predict prognosis (16,17). Anthracyclin has been also known to induce the degeneration of ventricular muscles and thus to show congestive heart failure features (5). Therefore, in this study, in patients administered anthracyclin chemotherapeutic agents, the correlation of the plasma concentration of BNP and cardiac complications was examined by measuring plasma BNP regularly.
The subjects were acute leukemia, malignant lymphoma and multiple myeloma cases admitted to the department of Hematooncology, Kosin University Gospel Hospital from March 2005 to March 2007, and treated with chemotherapy including anthracyclin. As age, the subjects were patients between 20 years and 65 years, and cases with the past history of cardiac diseases, patients with cor pulmonale, patients with brain metastasis, and patients with liver failure (serum ALP/AST > more than 2 times of normal values) were excluded. The follow up period was from 6 months to 2 years.
For trans-thoracic echocardiographic test, the sonos 5,500 equipment (Hewlett-Packard, Andover, Massachusetts) was used. As echocardiographic test, according to the method recommended by the American Society of Echocardiography, the M type, two dimensional echocardiography and Doppler echocardiography were performed, and by the M type echocardiography, the thickness of the ventricular septum, the thickness of the left ventricular posterior wall, the diameter of the left ventricle at the end-diastolic phase, the diameter of the left ventricle during the end-systolic phase, and the diameter of the right and left atrium were measured, and applying the modified Simpson method, the ejection fraction of the left ventricle was measured.
In all the subject patients, they were measured prior to each treatment cycle. The concentration of plasma BNP and troponin-I was measured by collecting the whole blood using a test tube containing ethylon deamine tetraacetic acid (EDTA). BNP plasma value was quantitatively examined by chemiluminescence immunoassay (CLIA) using the Triage® BNP kit, the lower limit of measurement was 5 pg/ml, and the normal maximal value was 100 pg/ml. Troponin-I was measured chemiluminescence immunoassay (CLIA) using the Beckman Coulter Access AccuTnI kit, the lower limit of measurement was 0 ng/ml, and the normal maximal value was 0.2 ng/ml.
For the statistical analysis of the data, the SPSS 14.0 program was used, continuous variables were presented as the median value, the upper limit and the lower limit, and non-continuous variables were presented as the frequency and percent. In univariate analysis, to examine the correlation of clinical factors including BNP with cardiac accident, continuous variables were analyzed using Mann-Whitney test, and non-continuous variables were analyzed using chi-square test. In multivariate analysis, factors showing the statistical correlation with P value less than 0.1 in univariate analysis were analyzed by logistic regression analysis, and factors showing P value less than 0.05 were defined to be statistically significant. To validate echocardiographic index mediating effects on plasma BNP concentration, correlation analysis was performed, and as the method of entered analysis, entered multiple regression analysis was used.
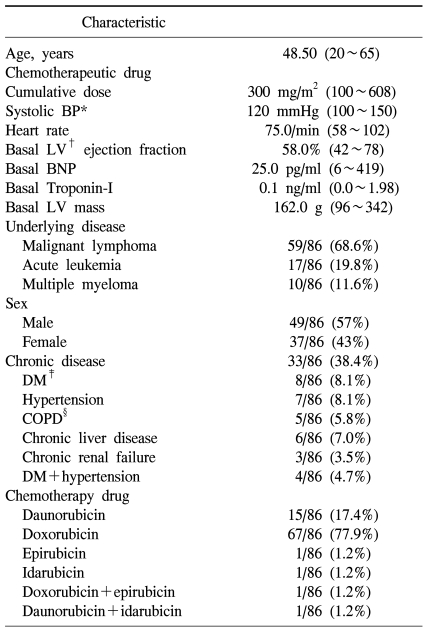
The subject patients were total 86 cases, their mean age was 48.5 years, and between 20 years and 65 years. Male patients were 49 cases (57%), and as underlying malignant diseases, malignant lymphoma patients were 59 cases (non-Hodgkins lymphoma was 52 patients, Hodgkins was 7 patients), which was most prevalent, acute leukemia was 17 patients, and multiple myeloma patient was 10 patients. Patients with associated chronic diseases were 33 patients of 86 patients (38.4%), patients accompanying hypertension were 7 cases, patients with diabetes were 8 cases, chronic hepatic disease was 6 cases, chronic renal failure was 3 cases, and patients with diabetes and hypertension simultaneously were 4 cases. In regard to anthracyclin chemotherapeutic agents administered, doxorubicin was 67 patients of 86 patients, which was used most prevalently, and in general, the cumulative anthracyclin dose was 300 mg/m2 (Table 1).
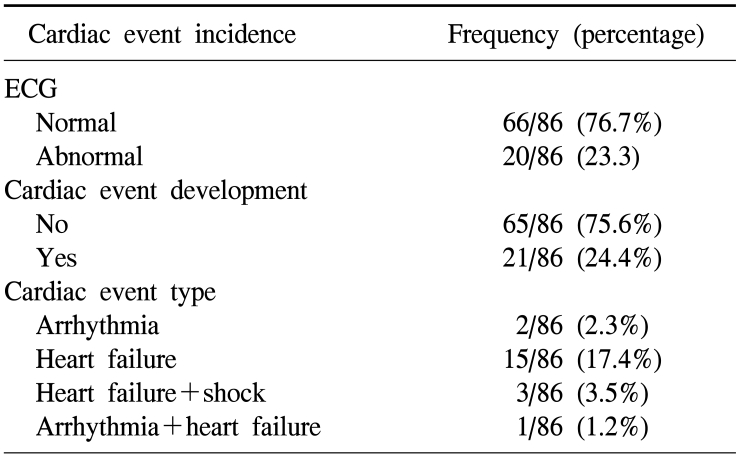
We defined the diagnosis of heart failure based on the Framingham diagnosis standard (it could be diagnosed when abnormality in more than one category among the major criteria such as nocturnal dyspnea, rale, cardiomegaly, acute pulmonary edema, cervical venous dilation, etc., and satisfy more than two minor criteria such as dyspnea during exercise, nocturnal cough, peripheral edema, hepatomegaly, pleural effusion, etc.25 Among the entire patients, cases whose electrocardigram was changed to abnormal were 20 patients (23.3%), cases developed cardiotoxicity were 21 patients (24.4%), cases showing only cardiac failure without symptoms of aneurysm or hypotension were 15 patients, which was most prevalent, and cases showing only aneurysm symptoms on electrocardiogram were 2 patients. Cases showing hypotension together with cardiac failure were 3 patients, and among them, 1 patient expired. Among 19 patients shown heart failure, all patients excluding 2 patients showed temporary heart failure, they were treated with diuretic and nitrate agents, and symptoms were improved within one month in most cases, and 2 patients showed chronic outcomes, and thus continuous treatments were required (Table 2).
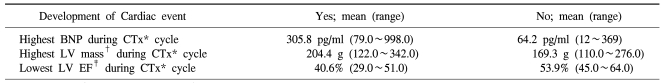
In the comparison of the maximal increased value of plasma BNP concentration of patients developed cardiotoxicity during the treatment of chemotherapy included Anthracyclin and other patients, in cases developed cardiotoxicity, the maximal value of BNP concentration was significantly elevated, and the maximal value of the left ventricular mass measured by echocardiography during the administration of anthracyclin was also significantly increased. The mean of the minimal value of left ventricular avascularization during chemotherapy of cases developed cardiotoxicity showed significantly lower values than cases without the development. Here, as cardiotoxicity accident, among the four types, heart failure, aneurysm, heart failure and shock, and heart failure and aneurysm, the subjects were cases developed only heart failure, because besides heart failure, the incidence of the cardiotoxicity accident aneurysm, shock, etc. were too low, and thus they were considered to be statistically not significant and excluded, and the result of the final analysis showed that plasma BNP concentration and left ventricular mass, and left ventricular avascularization rate were all showed a correlation to heart failure (Table 3).
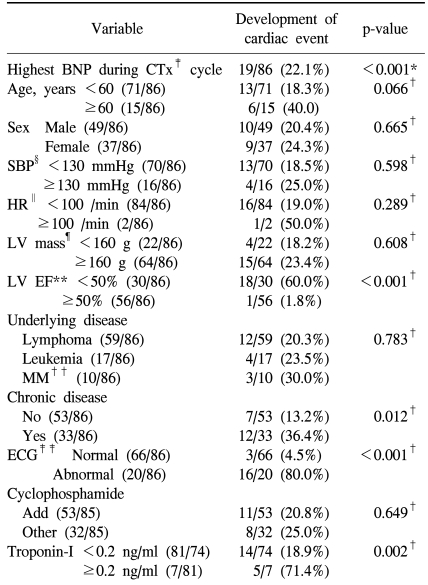
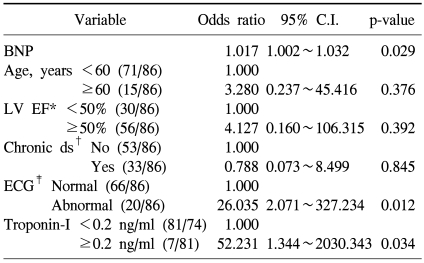
The development of cardiotoxicity based on the presence or absence of the development of aneurysm, heart failure, and shock was examined, and it was found to be developed in 21 patients (Table 2), and among them, 19 patients showed cardiac failure, and in univariate analysis and multivariate analysis, cardiotoxicity event developed during chemotherapy was limited to heart failure among the types of the development of cardiotoxicity. In univariate analysis, factors related to the development of heart failure were cases with the elevation of plasma BNP concentration (p<0.001), cases that ventricular avascularization rate was decreased to less than 50% (p<0.001), cases accompanying chronic diseases (p=0.012), cases showing abnormalities on echocardiogram (p<0.001), and cases that troponin-I was increased to more than 0.2 ng/ml (p=0.002). In addition, in cases older than 60 years, although it was not statistically significant, the possibility of the development of cardiotoxicity was shown to be high (p=0.066).
In multivariate analysis, as factors associated with the development of cardiotoxicity, the elevation of plasma BNP concentration, abnormal echocardiogram, and the elevation of troponin-I were confirmed to be statistically significantly associated.
Chemotherapeutic agents used for chemotherapy may cause permanent injury of normal tissues as well as diverse organs in human body, such side effects may become problems particularly in diseases that long term survival is possible, and even if malignant diseases were cured, due to sequelae impairment, it may cause the deterioration of quality of life. Particularly, the myocardium is formed by cells with limited regeneration ability, and thus if injured once, it shows permanent dysfunction, hence, if side effects of chemotherapy occurred, it could be considered to be fatal (1-8).
The effect of chemotherapeutic agents on the myocardium could be predicted, nonetheless, occasionally, it could not be predicted in some cases. The anthracyclin daunorubicin, doxorubicin, epirubicin, and idarubicin induce myocardial degeneration in association with the cumulative drug dose, and in such cases, it causes acute or subacute cardiotoxicity, and it may cause chronic complications (9).
Acute toxicity induced by anthracyclin chemotherapeutic agents are supraventricular tachycardia, ventricular tachycardia, pericarditis, severe changes of electrocardiogram, and myocardiopathy, nevertheless, such acute toxicity appears temporarily, and if treated, it could be recovered completely without chronic outcomes. 6 In subacute cardiotoxicity cases, it may be developed between 3 months and 8 months after the completion of chemotherapy, and it shows heart failure features primarily. In chronic cardiotoxicity, it is developed after 5 years, left ventricular function is deteriorated gradually leading to congestive heart failure, and afterward, it lasts for a long time, and thus quality of life is lowered (2,3,7,11). Therefore, it could be considered to be important to recognize the high risk patient group who may develop cardiotoxicity induced by chemotherapeutic agents, and to prevent and treat it.
Ventriculography applying echocardiogram and radioactive isotopes is a non-invasive test, and it has been applied widely to the examination of the cardiotoxicity induced by chemotherapeutic agents, nevertheless, as a test applied to the early detection of condition that cardiac condition is not completely deteriorated yet, its sensitivity is low (10).
It has been well known that plasma concentration of Brain Natriuretic Peptide (BNP) that is a type of cardiac neuronal hormone released from the ventricles by the elevation of ventricular pressure correlates significantly to the end diastolic pressure of the left ventricle and the wedge pressure of pulmonary capillary blood vessels in heart failure patients, and it reflects the dysfunction of the left ventricle pertinent to it. The avascularizatin rate of the left ventricle applying echocardiogram is an important means to observe and monitor heart failure, and it is good for the diagnosis of heart failure that has been developed already, however, it is not useful for the prediction of early cardiotoxicity. Not only plasma BNP concentration could be measured rapidly and readily, even in cases with normal avascularization rate, if the concentration were elevated, the increase of ventricular load could be suspected, and thus it could be applied as a factor that could predict early cardiotoxicity (14-19). In our study, among 86 patients, more than 60% were malignant lymphoma patients, and they received the combination chemotherapy of cyclophosphamide, vincristine, and prednisolon including anthracyclin. In adults, malignant hematological diseases are developed in relatively older patients, between the age of 50 years and 70 years, and in such cases, many patients have chronic diseases such as hypertension, diabetes, etc (12). As shown in the results, the presence or absence of accompanying chronic disease acts as an independent risk factor in the development of cardiac complications. Particularly, in the treatment of non-Hodgkins lymphoma, most cases receive combination chemotherapy including steroids such as prednisolon, and the incidence of secondary diabetes has been reported to be 10~20%, and thus it may increase the risk of the development of cardiac diseases.
Cardiac troponin has been shown to be the most sensitive and specific factor for the prediction of myocardial injury, and its usefulness is applied not only to the diagnosis of acute myocardial infarction, but also to predict the short term or long term risk level of acute coronary artery syndrome (20,21). According to some studies, in cardiotoxicity associated with the use of anthracyclin chemotherapeutic agents, serum troponin-I value has been shown to be related to the histopathological change of the myocardium, and particularly, its elevation persistent for longer than one monthe is associated not only with cardiac dysfunction that would be developed in the future, but also more serious cardiac complications (21). In our study, similarly, cardiotoxicity developed after chemotherapy showed a correlation to troponin-I.
Adriamycin and cyclophosphamide are durgs of which cardiotoxicity is well known, and recently, for cases older than 60 years, with the history of the previous use of anthracyclin, or with chronic diseases pentinent to the heart, the trend is to use liposome-encapsulated doxorubicin of which cardiotoxicity is less, and consequently, cardiotoxicity could be reduced (22). Particularly, multiple myeloma is a disease of which onset is between the late 60s and the 70s preferentially, and most patients have risk factors of cardiotoxicity, hence, attentive monitoring of the heart may be necessary.
According to the research reports performed by numerous prospective as well retrospective analysis, cardiotoxicity appears frequently in cases whose cumulative dose of anthracyclin chemotherapeutic agents is higher than 300 mg/m2 or with previous cardiac diseases (4). However, in our study, in the comparison of cases whose cumulative dose was higher than 300 mg/m2 with cases lower than that, the incidence of cardiotoxicity did not show a statistical difference, and among the entire 86 patients, cases whose cumulative dose was higher than 500 mg/m2 were 6 patients, and cardiotoxicity was developed in 4 patients, and thus the correlation to cardiotoxicity was detected in cases whose cumulative dose of anthracyclin chemotherapeutic agents was higher than 500 mg/m2.
Although not all oncologists agree, in many cases, the use of liposome type anthracyclin derivatives for patients with risk factors has been accepted and recommended (23). In several reports, it has been reported that cardiotoxicity could be reduced by the use of dextrazoxane, and iron chylators, hence, in cases using anthracyclin chemotherapeutic agents from the initial time, its use was recommended from the prophylactic aspect, nonetheless, large numbers recommend to use it on limited cases with the cumulative dose higher than 300 mg/m2 (24).
Based on our results, in malignant hematological tumor patients whose long term survival is possible, for cases receiving chemotherapy including anthracyclin chemotherapeutic agents, the elevation of the concentration or value through the regular measurement of plasma BNP concentration and troponin-I may correlate to the development of cardiotoxicity, and if their abnormal findings were detected, to perform aggressive prevention and treatment for the heart may be of help to improve the long term morbidity and survival of malignant diseases of which complete cure is anticipated, and furthermore, to improve quality of life.
In patients received chemotherapy including anthracyclin chemotherapeutic agents, the elevation of serum BNP concentration is a factor associated with the development of cardiotoxicity, and to understand the more accurate cause-effect of the correlation of serum BNP concentration and the development of cardiotoxicity, it is considered that prospective clinical studies are required in the future.
References
1. Floyd JD, Nguyen DT, Lobins RL, Bashir Q, Doll DC, Perry MC. Cardiotoxicity of cancer therapy. J Clin Oncol. 2005; 23:7685–7696. PMID: 16234530.

2. Ewer M, Benjamin RS. Perry MC, editor. Cardiotoxicity of chemotherapeutic drugs. The chemotherapy sourcebook. 2001. 3rd ed. Philadelphia, PA: Lippincott: Williams & Wilkins;p. 458–468.
3. Bonneterre J, Roche H, Kerbrat P, Fumoleau P, Goudier MJ, Fargeot P, et al. Long-term cardiac follow-up in relapse-free patients after six courses of fluorouracil, epirubicin, and cyclophosphamide, with either 50 or 100 mg of epirubicin, as adjuvant therapy for node-positive breast cancer: French Adjuvant Study Group. J Clin Oncol. 2004; 22:3070–3079. PMID: 15284257.

4. Poutanen T, Tikanoja T, Riikonen P, Silvast A, Perkkio M. Long-term prospective follow-up study of cardiac function after cardiotoxic therapy for malignancy in children. J Clin Oncol. 2003; 21:2349–2356. PMID: 12805337.

5. Hequet O, Le QH, Moullet I, Pauli E, Salles G, Espinouse D, et al. Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J Clin Oncol. 2004; 22:1864–1871. PMID: 15143078.

6. Bristow MR, Thompson PD, Martin RP, Mason JW, Billingham ME, Harrison DC. Early anthracycline cardiotoxicity. Am J Med. 1978; 65:823–832. PMID: 707541.

7. Steinherz LJ, Yahalom J. DeVita VT, Hellman S, Rosenberg SA, editors. Cardiac toxicity. Cancer: principles and practice of oncology. 2001. 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins;p. 2904–2921.
8. Theodoulou M, Seidman AD. Cardiac effects of adjuvant therapy for early breast cancer. Semin Oncol. 2003; 30:730–739. PMID: 14663774.
9. Nysom K, Holm K, Lipsitz SR, Mone SM, Colan SD, Orav EJ, et al. Relationship between cumulative anthracycline dose and late cardiotoxicity in childhood acute lymphoblastic leukemia. J Clin Oncol. 1998; 16:545–550. PMID: 9469339.

10. Ganz WI, Sridhar KS, Ganz SS, Gonzalez R, Chakko S, Serafini A. Review of tests for monitoring doxorubicin-induced cardiomyopathy. Oncology. 1996; 53:461–470. PMID: 8960141.
11. Hale ER, Lipshultz S, Constine L. Latent injury after the double-edged sword of chemo/radiation. 2005 ASCO educational book. 2005. Alexandria, VA: American Society of Clinical Oncology;p. 739–746.
12. Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979; 91:710–717. PMID: 496103.
13. Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995; 332:1738–1743. PMID: 7760889.

14. Maisel AS, Koon J, Krishnaswamy P, Kazenegra R, Clopton P, Gardetto N, et al. Utility of B-natriuretic peptide as a rapid, point-of-care test for screening patients undergoing echocardiography to determine left ventricular dysfunction. Am Heart J. 2001; 141:367–374. PMID: 11231433.

15. Hayakawa H, Komada Y, Hirayama M, Hori H, Ito M, Sakurai M. Plasma levels of natriuretic peptides in relation to doxorubicin-induced cardiotoxicity and cardiac function in children with cancer. Med Pediatr Oncol. 2001; 37:4–9. PMID: 11466716.

16. Nousiainen T, Vanninen E, Jantunen E, Remes J, Ritanen E, Vuolteenaho O, et al. Neuroendocrine changes during the evolution of doxorubicin-induced left ventricular dysfunction in adult lymphoma patients. Clin Sci (Lond). 2001; 101:601–607. PMID: 11724646.

17. Nousiainen T, Jantunen E, Vanninen E, Remes J, Vuolteenaho O, Hartikainen J. Natriuretic peptides as markers of cardiotoxicity during doxorubicin treatment for non-Hodgkin's lymphoma. Eur J Haematol. 1999; 62:135–141. PMID: 10052718.

18. Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005; 23:7820–7826. PMID: 16258084.

19. Lipshultz SE, Colan SD. Cardiovascular trials in long-term survivors of childhood cancer. J Clin Oncol. 2004; 22:769–773. PMID: 14990630.

20. Herman EH, Lipshultz SE, Rifai N, Zhang J, Papoian T, Yu ZX, et al. Use of cardiac troponin T levels as an indicator of doxorubicin-induced cardiotoxicity. Cancer Res. 1998; 58:195–197. PMID: 9443390.
21. Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000; 36:517–522. PMID: 10933366.

22. Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001; 19:1444–1454. PMID: 11230490.

23. Speyer JL, Green MD, Kramer E, Rey M, Sanger J, Ward C, et al. Protective effect of the bispiperazinedione ICRF-187 against doxorubicin-induced cardiac toxicity in women with advanced breast cancer. N Engl J Med. 1988; 319:745–752. PMID: 3137469.

24. Swain SM, Whaley FS, Gerber MC, Ewer MS, Bianchine JR, Gams RA. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. J Clin Oncol. 1997; 15:1333–1340. PMID: 9193324.

25. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971; 285:1441–1446. PMID: 5122894.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download