Abstract
Purpose
Metastatic breast cancer patients are usually exposed to taxane and anthracycline as neoadjuvant, adjuvant and palliative chemotherapeutic agents. This study was designed to determine the efficacy and safety of the use of a gemcitabine and cisplatin (GP) combination treatment in patients with metastatic breast cancer that were pretreated with anthracycline and taxane.
Materials and Methods
We evaluated the use of a GP regimen (1,000 mg/m2 gemcitabine administered on days 1 and 8 plus 60 mg/m2 cisplatin administered on day 1 every 3 weeks) in 38 breast cancer patients who had received prior chemotherapy with anthracycline and taxane as an adjuvant or neoadjuvant therapy, or as a palliative therapy.
Results
The median patient age was 49 years (age range, 35~69 years). The overall response rate was 28.9% in 11 patients (95% confidence interval [CI], 14~44%). The median time to progression was 5.2 months (95% CI, 3.6~6.8 months). Median survival was 19.5 months (95% CI, 11.2~27.8 months). Major grade 3/4 hematological toxicity was due to leukopenia (36 of 157 cycles, 23.1%). Non-hematological toxicity was rarely severe; grade1/2 nausea and vomiting were observed in 37.8% of the patients. There were no treatment related deaths.
Go to : 
Breast cancer is a major cancer of women worldwide and the incidence of the disease has been increasing annually. For the past several years, breast cancer has been the most prevalent cancer among Korean women; the annual incidence may now exceed 10,000 cases (1). It has been estimated that metastatic breast cancer develops in 35~40% of all patients with breast cancer (2).
Metastatic breast cancer patients are usually exposed to anthracycline and taxane as neoadjuvant, adjuvant and palliative chemotherapeutic agents. Although anthracycline and the taxane are the most active first-line drugs for treatment, the cancers of many patients will progress and require the use of other chemotherapeutic agents (3). Among several agents that have been used, gemcitabine and platinum compounds have been well-characterized single agents (4,5), but there are few studies about the use of a combination of these two types of chemotherapeutic agents. Synergism between gemcitabine (a compound that inhibits DNA repair) and cisplatin (a compound that induces DNA damage) has been demonstrated in in vitro studies (6). Exposure to gemcitabine can counteract the cisplatin resistance that results from the up-regulation of DNA repair processes. Cisplatin enhances the rate of incorporation of gemcitabine, leading to apoptosis (7). Clinically, these agents have partially non-overlapping toxicity, as gemcitabine does not enhance cisplatin-induced nephrotoxicity or neurotoxicity, and cisplatin causes only mild myelotoxicity (8).
We have conducted this prospective phase II study in an effort to evaluate the efficacy and safety of the use of gemcitabine and cisplatin (GP) combination chemotherapy in patients with metastatic breast cancer that were pretreated with anthracycline and taxane.
Go to : 
To be eligible for this study, patients were required to have a histologically confirmed diagnosis of carcinoma of the breast and at least one measurable lesion. This study included patients who had received anthracycline-based and taxane-based previous neoadjuvant, adjuvant and palliative chemotherapy regimens. Only patients with Eastern Cooperative Oncology Group (ECOG) performance status of grade 0~2 were enrolled in this study. The patients had no active infections, no serious or uncontrolled concurrent medical illness and no previous history of other malignancies. Adequate hepatic, renal and bone marrow function was essential. The local ethics committee approved the study and informed consent was obtained from all patients before study entry.
Chemotherapy was administered through a chemoport placed in the subclavian vein or directly into a peripheral vein. The patients were administered 1,000 mg/m2 gemcitabine (1-hour infusion) on days 1 and 8, and the patients were administered 60 mg/m2 cisplatin (over a 1-hour infusion) on day 1. Each cycle of chemotherapy was given every 3 weeks if the patient blood count had returned to an acceptable level (WBC: 3,000×103/µl; platelets: 70,000×103/µl) and non-hematological toxic effects had resolved. The dosage of the subsequent cycles was adjusted according to the toxic effects that developed during the preceding cycle. If the hematological values were not reached by the date of the scheduled retreatment, therapy was delayed in weekly intervals, and if the hematological criteria were not fulfilled after a delay of two weeks, the patient was removed from the study. Doses of gemcitabine and cisplatin were reduced by 25% during subsequent cycles if the neutrophil and platelet count nadirs were <500×103/µl or <50,000×103/µl, respectively, or if neutropenic fever developed. In addition, on chemotherapy day 8, a complete bloodc count was performed before gemcitabine treatment. The administration of gemcitabine was omitted in cases of grade 4 hematologic toxicity on day 8. The primary endpoint of this study was the tumor response rate and secondary endpoints included time to progression (TTP), overall survival and toxicities.
All patients received a standard supportive regimen consisting of hydration with normal saline for at least 3 L/24 hours, dexamethasone and the use of 5-HT3 inhibitors. Follow-up history and physical examinations, tumor measurements, and toxicity assessments were performed before each 3-week cycle of therapy. Toxicity was assessed using the National Cancer Institute-Common Toxicity Criteria (NCI-CTC), Version 3.0.
A physical examination, complete blood counts, blood chemistry and chest x-rays were obtained after each cycle. Response was assessed using WHO criteria (9). Computed tomography (CT) scans were repeated every three cycles or earlier in cases of clinical deterioration.
This trial was designed to detect a response rate of 30% as compared to a minimal, clinically meaningful response rate of 10%. A two-stage optimal design as proposed by Simon was adopted, with a statistical power of 80% to accept the hypothesis and 5% significance to reject the hypothesis. Allowing for a follow-up loss rate of up to 20%, the total sample size required was 35 patients with measurable disease. The time to progression (TTP) and overall survival (OS) were calculated from the initiation of treatment to the first observation of disease progression or death, respectively. All data were analyzed using SPSS software (version 12.0, Chicago-IL). Prognostic factors for OS, TTP and response rate (RR) were analyzed by use of Fisher's exact test, Kaplan-Meier and Cox regression analyses.
Go to : 
Between November 2002 and July 2007, 38 patients were assigned to be treated at the Department of Internal Medicine at Dong-A University Medical Center, Busan, South Korea. The characteristics of the 38 patients enrolled in this study are described in Table 1. The median patient age was 49 years (age range, 35~69 years). More than half of the 38 patients (21 patients, 55.3%) were premenopausal. The performance status (ECOG) was grade 0-1 in most of patients; nine patients (23.7%) were grade 2. In 24 patients (63.2%), the hemoglobin level was 12 g/dl. The tumor marker CA15-3 was elevated in 13 patients (34.2%). For the hormonal receptor status, expression of estrogen receptor (ER) and/or progesterone receptor (PR) was positive in 22 (57.9%) patients; HER2 was positive in 11 (28.9%) patients. A total of 33 (86.8%) patients had received prior palliative chemotherapy of more than one regimen. For metastasis, there was one involved organ in five patients (13.2%), two involved organs in 12 patients (31.6%) and three or more involved organs in ten patients (26.3%). The lung was the most common metastatic site (21 patients, 55.3%), and lymph nodes (19 patients, 50.0%), the liver (ten patients, 26.3%), bone (ten patients, 26.3%) and the skin and soft tissue (nine patients, 23.7%) were multiply involved.
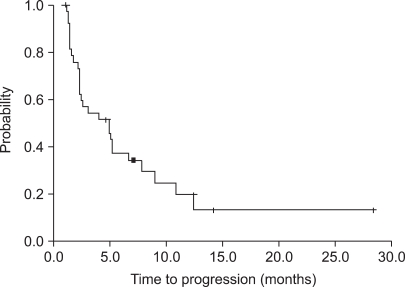
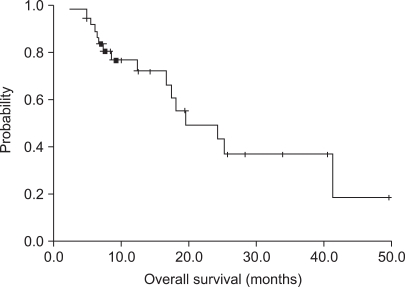
Of the 38 patients who were assessed for response, a partial response (PR) was seen in 11 patients (28.9%; 95% confidence interval [CI], 14~44%), stable disease (SD) was seen in 14 patients (36.8%) and progressive disase (PD) was seen in 13 patients (34.2%). The median follow-up duration was 25.7 months. The median TTP was 5.2 months (95% CI; 3.6~6.8 months) (Fig. 1). The median OS was 19.5 months (95% CI; 11.2~27.8 months) (Fig. 2).
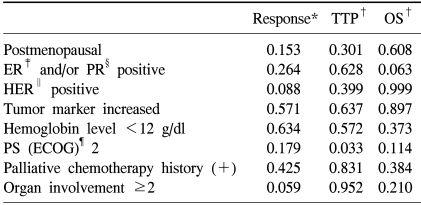
Only ECOG performance status 2 was a poor prognostic factor for TTP (p=0.033). Other variations of patient characteristics were not significant prognostic factors for the response rate, TTP and OS (Table 2).
In case of disease progression with the use of gemcitabine and cisplatin chemotherapy, salvage chemotherapy was permitted and was performed in 22 of 25 patients with the use of various drugs. Trastuzumab was used in seven of 11 patients that were positive for ER2 expression. A response to salvage treatment was observed in eight patients (32%).
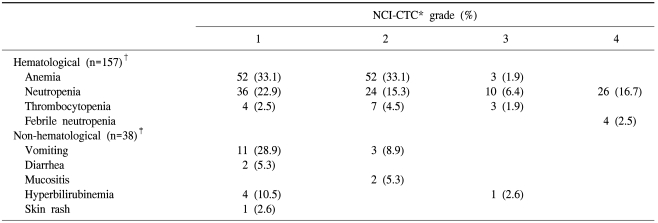
A total of 157 treatment cycles were administrated to the 38 patients with a median number of four cycles (range, 1~10 cycles). The number of dose reduction cycles were 38 (24%) among the 157 cycles. The most common reason for dose reduction was neutropenia (74%), followed by thrombocytopenia (16%) and hyperbilirubinemia (10%). The relative dose intensities were 91% for gemcitabine and 93% for cisplatin.
An analysis of side effects showed that the toxicities were in general manageable and that anemia (grade 1 or 2 in 66.2% or 104 of 157 cycles) and neutropenia (grade 1 or 2 in 38.2% or 60 of 157 cycles) were the main toxicities. Grade 3 or 4 neutropenia were observed in 22.9% or 36 cycles, but only four episodes of non-fatal neutropenic fever were observed. Grade 1 or 2 vomiting was observed in 14 patients (37.8%), and diarrhea (grade 1 in two patients), mucositis (grade 2 in two patients) and hyperbilirubinemia (grade1 or 3 in five patients) were reported. A grade 1 skin rash was observed in one patient. There were no treatment-related deaths (Table 3).
Go to : 
Anthracycline and taxane usually have been used for metastatic breast cancer as a first or second line treatment, but many patients have disease progression and require the use of other chemotherapeutic agents. Vinorelbine, gemcitabine, cisplatin and capecitabine in single or combination regimens have been used, but there are no reported significant differences in the RR, TTP and OS.
Gemcitabine has been used as a single agent in the treatment of metastatic breast cancer (10-13). In a first-line setting, the response rates were 14~37% (10,13). When conducted in primarily pretreated patients, reproducible response rates were 17~29% (11,12). When a platinum compound was used as a single agent for metastatic breast cancer, the response rates were 32~50% in first-line therapy and approximately 10% in a salvage setting (14).
In this study, an RR of 28.9%, a median survival of 19.5 months and a median TTP of 5.2 months were achieved. Previously reported treatment response rates of gemcitabine-cisplatin combination chemotherapy have been variable, ranging from 26~80% (2,8,15,16). For patients with metastatic breast cancer pretreated with anthracycline and taxane, a response rate of 34.3%, a median OS of 13.5 months and a TTP of 6.0 months have been reported (15). As compared with the findings in the present study, a slightly higher RR and TTP have been reported, and these findings may be due to a lower proportion (50%) of the use of greater than third-line salvage chemotherapy than in our study (68.4%). In addition, a lower proportion of patients with a positive HER 2 status (22% versus 28.9%) and a higher proportion of patients with a positive ER and/or PR status (95% versus 57.9%) may be contributing to a higher RR and TTP than in the present study.
For the use of other palliative chemotherapy regimens, the response rates that were observed include the following. For the use of gemcitabine-vinorelbine, an RR of 22~55.5% and a TTP of 6.8~9.5 months for first-line and second line treatments have been reported (17-19). For the use of capecitabine, an RR of 28% and a TTP of 4.9 months for second-line to fifth-line treatment have been reported (20). For the use of gemcitabine-docetaxel, an RR of 36~79% and a TTP of 7~8 months for first-line to third-line treatment have been reported (21-23). For the use of gemcitabine-paclitaxel, an RR of 55~66.7% and a TTP of 11 months for first-line to third-line treatment have been reported (24,25). For the use of gemcitabine-paclitaxel-trastuzumab, an RR of 52.5% and a TTP of 13.7 months for first-line treatment for HER2-positve cases have been reported (20).
A reason for a lower response rate in our study might be that 68.4% (26 patients) of all of the patients had undergone a GP regimen as more than a third-line salvage treatment. Although the response rate was lower than reported in previous studies, the TTP was comparable with the results of previous studies. A higher overall survival (19.5 months) may be due to the use of salvage chemotherapy. The RR of treatment after progression was also similar for the GP regimen. Follow-up was performed for a short duration in many patients (less than six months for 13 patients; 34%), and data that was censored data was increased for OS and TTP (Fig. 1, 2).
Neutropenia, anemia, nausea, and vomiting were the main toxicities, the same findings as reported in a previous study. Most of these toxicities were Grade 1 or 2, and were usally manageable.
For the prognostic factors, positive expression of HER-2 was not a significant prognostic factor for survival. This may be due to the use of trastuzumab in only seven patients (7/11, 63.6%) of the positive HER-2 patients. Only performance status was a significant prognostic factor for TTP.
Most of the chemotherapy studies of metastatic breast cancer are phase II studies, and the benefit and harm of these therapies have not yet been confirmed. In our study, performance status was the only notable prognostic factor. Therefore, prior to the initiation of chemotherapy, it is necessary to evaluate the performance status to achieve a benefit for the patients.
Go to : 
References
1. Shin HR, Jung KW, Won YJ, Park JG. 2002 annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004; 36:103–114.

2. Wirk B, Perez E. Role of gemcitabine in breast cancer management: an update. Semin Oncol. 2006; 33:S6–S14. PMID: 16472712.

4. Seidman AD. Monotherapy options in the management of metastatic breast cancer. Semin Oncol. 2003; 30(2 Suppl 3):6–10. PMID: 12722018.

5. Heinemann V. Role of gemcitabine in the treatment of advanced and metastatic breast cancer. Oncology. 2003; 64:191–206. PMID: 12697958.

6. van Moorsel CJ, Veerman G, Bergman AM, Guechev A, Vermorken JB, Postmus PE, et al. Combination chemotherapy studies with gemcitabine. Semin Oncol. 1997; 24:S7-17–S7-23. PMID: 9194475.
7. Achanta G, Pelicano H, Feng L, Plunkett W, Huang P. Interaction of p53 and DNA-PK in response to nucleoside analogues: potential role as a sensor complex for DNA damage. Cancer Res. 2001; 61:8723–8729. PMID: 11751391.
8. Nagourney RA, Link JS, Blitzer JB, Forsthoff C, Evans SS. Gemcitabine plus cisplatin repeating doublet therapy in previously treated, relapsed breast cancer patients. J Clin Oncol. 2000; 18:2245–2249. PMID: 10829044.

9. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47:207–214. PMID: 7459811.

10. Blackstein M, Vogel CL, Ambinder R, Cowan J, Iglesias J, Melemed A. Gemcitabine as first-line therapy in patients with metastatic breast cancer: a phase II trial. Oncology. 2002; 62:2–8. PMID: 11810037.

11. Brodowicz T, Kostler WJ, Moslinger R, Tomek S, Vaclavik I, Herscovici V, et al. Single-agent gemcitabine as second- and third-line treatment in metastatic breast cancer. Breast. 2000; 9:338–342. PMID: 14965758.

12. Spielmann M, Llombart-Cussac A, Kalla S, Espie M, Namer M, Ferrero JM, et al. Single-agent gemcitabine is active in previously treated metastatic breast cancer. Oncology. 2001; 60:303–307. PMID: 11408796.

13. Possinger K, Kaufmann M, Coleman R, Stuart NS, Helsing M, Ohnmacht U, et al. Phase II study of gemcitabine as first-line chemotherapy in patients with advanced or metastatic breast cancer. Anticancer Drugs. 1999; 10:155–162. PMID: 10211545.

14. Martin M. Platinum compounds in the treatment of advanced breast cancer. Clin Breast Cancer. 2001; 2:190–208. PMID: 11899413.
15. Heinemann V, Stemmler HJ, Wohlrab A, Bosse D, Losem C, Kahlert S, et al. High efficacy of gemcitabine and cisplatin in patients with predominantly anthracycline- and taxane-pretreated metastatic breast cancer. Cancer Chemother Pharmacol. 2006; 57:640–646. PMID: 16163537.

16. Smith IE. Overview of gemcitabine activity in advanced breast cancer. Semin Oncol. 2006; 33(3 Suppl 9):S19–S23. PMID: 16797378.

17. Valenza R, Leonardi V, Gebbia V, Agostara B. Gemcitabine and vinorelbine in pretreated advanced breast cancer: a pilot study. Ann Oncol. 2000; 11:495–496. PMID: 10847474.

18. Stathopoulos GP, Rigatos SK, Pergantas N, Tsavdarides D, Athanasiadis I, Malamos NA, et al. Phase II trial of biweekly administration of vinorelbine and gemcitabine in pretreated advanced breast cancer. J Clin Oncol. 2002; 20:37–41. PMID: 11773151.

19. Nicolaides C, Dimopoulos MA, Samantas E, Bafaloukos D, Kalofonos C, Fountzilas G, et al. Gemcitabine and vinorelbine as second-line treatment in patients with metastatic breast cancer progressing after first-line taxane-based chemotherapy: a phase II study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol. 2000; 11:873–875. PMID: 10997817.

20. Fountzilas G, Christodoulou C, Tsavdaridis D, Kalogera-Fountzila A, Aravantinos G, Razis E, et al. Paclitaxel and gemcitabine, as first-line chemotherapy, combined with trastuzumab in patients with advanced breast cancer: a phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG). Cancer Invest. 2004; 22:655–662. PMID: 15581045.

21. Fountzilas G, Nicolaides C, Bafaloukos D, Kalogera-Fountzila A, Kalofonos H, Samelis G, et al. Docetaxel and gemcitabine in anthracycline-resistant advanced breast cancer: a Hellenic Cooperative Oncology Group Phase II study. Cancer Invest. 2000; 18:503–509. PMID: 10923097.

22. Laufman LR, Spiridonidis CH, Pritchard J, Roach R, Zangmeister J, Larrimer N, et al. Monthly docetaxel and weekly gemcitabine in metastatic breast cancer: a phase II trial. Ann Oncol. 2001; 12:1259–1264. PMID: 11697837.

23. Alexopoulos A, Tryfonopoulos D, Karamouzis MV, Gerasimidis G, Karydas I, Kandilis K, et al. Evidence for in vivo synergism between docetaxel and gemcitabine in patients with metastatic breast cancer. Ann Oncol. 2004; 15:95–99. PMID: 14679126.

24. Murad AM. Paclitaxel and gemcitabine as salvage treatment in metastatic breast cancer. Oncology (Williston Park). 2003; 17(12 Suppl 14):26–32. PMID: 14768402.
25. Delfino C, Caccia G, Gonzales LR, Mickiewicz E, Rodger J, Balbiani L, et al. Gemcitabine plus paclitaxel as first-line chemotherapy for patients with advanced breast cancer. Oncology. 2004; 66:18–23. PMID: 15031594.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download