Abstract
Rituximab is a human/murine chimeric anti-CD20 monoclonal antibody used to treat CD20-positive B-cell non-Hodgkin's lymphoma (NHL). Although most of the adverse effects associated with rituximab are usually reversible and temporary infusion-related reactions, including fever, chills, flushing and skin reactions, there are several reports of pulmonary events after long-term administration of rituximab. We present a case of asymptomatic nodular organizing pneumonia occurring during rituximab-based chemotherapy in a patient with non-Hodgkin's lymphoma.
Rituximab is one of the most widely accepted treatment methods for diffuse large B-cell non-Hodgkin's lymphoma. Rituximab is a human/murine chimeric monoclonal antibody that targets B lymphocytes expressing CD20 and mediates complement- and antibody-mediated cytotoxic effects. Since the combination of rituximab with CHOP, which has been used as a treatment for diffuse large B-cell lymphoma, showed a significant survival benefit as well as complete response rate, this combination regimen has been considered to be the standard treatment for diffuse non-Hodgkin's lymphoma expressing CD20 (1,2). The adverse side effects associated with the administration of rituximab are usually transient infusion-related reactions, including fever, chills, myalgia, sweating, and skin rash, and often improve spontaneously. Although some of its side effects, such as bronchospasm, hypotension, or angioedema, could be often fatal, the incidence of these side effects is extremely rare and transient (3,4). However, recent reports have indicated that the rates of respiratory side effects, including interstitial pneumonia, bronchiolitis obliterans organizing pneumonia (BOOP), and acute pulmonary fibrosis, occurring during the prolonged follow-up period after rituximab therapy have been on the rise (5~10). We report a 66-year-old man with organizing pneumonia occurring during treatment with rituximab-containing combination chemotherapy for non-Hodgkin's lymphoma.
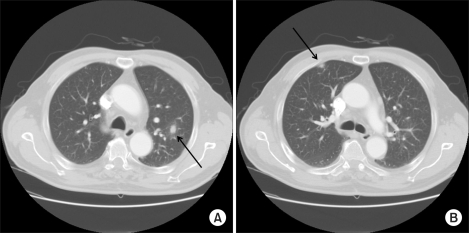
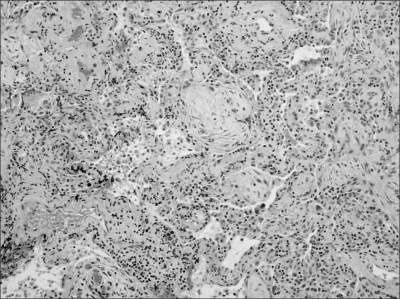
A 66-year-old man presented with a left cervical mass, abdominal pain, and weight loss and was admitted to our hospital. Paraaortic, aortocaval, and mesenteric root lymphadenopathies were identified on abdominal CT scan. Biopsy from the left cervical mass confirmed non-Hodgkin's lymphoma of the CD20-positive diffuse large B-cell type. There was no bone marrow infiltration. LDH was elevated at 1,916 IU/L with Ann Arbor stage IIIB and ECOG performance status 0, resulting in an International Performance Index grade of high-intermediate. The patient received combination chemotherapy with rituximab and CHOP. There were no acute side reactions associated with rituximab, nor was there any febrile neutropenia or any evidence of infection. From the fourth cycle on, granulocyte colony-stimulating factor was administered at the end of each cycle due to febrile neutropenia. PET/CT and abdominal CT scans were performed one month after the completion of six cycles of chemotherapy and revealed the disappearance of the lymphadenopathies identified on the previous examinations, except for one residual hypermetabolic lesion around the transverse colon shown on the PET/CT scan. The sizes of all measurable lesions were less than 1 cm. There were, however, two newly developed nodular hot-uptakes in the upper lobes of both lungs on PET/CT scan (Fig. 1). These lesions also appeared as ill-defined increased opacities on chest X-ray. Chest CT scan demonstrated bilateral nodular lesions, 1-cm in diameter, in the upper lobes (Fig. 2). Biopsy of the lesion on the right upper lobe was performed via video-assisted thoracoscopy since the pulmonary lesions had relatively clear margins and were suspected to suggest the possibility of pulmonary involvement in lymphoma. Unexpectedly, the pathologic finding was rather consistent with organizing pneumonia, showing distal alveolar spaces full of organizing fibrosis (fibroblastic foci) with uniform temporal appearance and mild interstitial fibrosis (Fig. 3). The patient was subjected to close observation due to the absence of respiratory symptoms and the lack of abnormalities on physical examination. Follow-up chest CT scan revealed spontaneous regression of the residual lesions in both upper lobes. The chest X-ray results remained unremarkable without any respiratory symptoms, and the patient is currently under observation.
Organizing pneumonia is a pathologic condition defined as a distal intra-alveolar space filled with fibroblasts and myofibroblasts with a connective tissue-forming granulomatous pattern. The etiology of organizing pneumonia is often idiopathic and can develop as the result of infection, drug intake or underlying disease (11). Cryptogenic organizing pneumonia has also been previously referred to as idiopathic bronchiolitis obliterans organizing pneumonia, and these disease entities are often used together with organizing pneumonia (12,13). Organizing pneumonia associated with rituximab is rare and mostly reported as case presentations. In fact, the well-known side effects of rituximab are likely to be transient infusion-related hypersensitivity reactions mediated by cytokines and are most prominent at the time of initial administration. The results of several current studies suggest that the repeated administration of rituximab could cause rituximab-induced organizing pneumonia (5~9). According to Alexnadrescu et al, the activation of a complement system and various cytokines, such as TNF-α, interleukin (IL)-6 and IL-8, probably plays a significant role in the pathophysiology of this disease (8,14). In addition, intracellular peptides released after cell lysis are known to make dendritic cells under maturation, activate cytotoxic T lymphocytes and eventually mediate delayed cytotoxic responses (8,14). This serial process may lead to the positive correlation between organizing pneumonia and repetitive administration of rituximab. A number of reports have indicated that the application of granulocyte-colony-stimulating factor (G-CSF) is one of the causes of organizing pneumonia after rituximab. Nevertheless, it remains unclear whether this case of organizing pneumonia was caused solely by rituximab or whether G-CSF played a role by strengthening the side effects of rituximab (6,8). Another distinct clinical presentation of this case is that focal organizing pneumonia presented as a solitary nodule or mass. Organizing pneumonia is usually classified into three subtypes based on its radiographic pattern: the multiple patchy migratory pulmonary involvement of pneumonia type, solitary pulmonary involvement of pneumonia type or the diffuse pulmonary involvement of the interstitial lung disease type (11,15).
Pulmonary nodular pattern may present as an asymptomatic mass often in the upper lung zone and can be treated by surgical resection (11). In this case, two nodules were identified in each upper lobe, respectively. Since this finding suggested the possibility of either primary lung cancer or pulmonary involvement in lymphoma, VATS biopsy was performed on the lesion of the right upper lobe. After several months of close observation, the spontaneous resolution of both lesions was confirmed by chest CT scan. The patients in the majority of reports concerning rituximab-induced organizing pneumonia were symptomatic and improved with steroid therapy; however, the patient in our case had no respiratory symptoms and improved spontaneously (5,6,8,9). The efficacy of corticosteroid therapy for asymptomatic organizing pneumonia remains controversial.
The various adverse effects of rituximab require a more-detailed and well-established study as the use of the drug is becoming increasingly more widespread in clinical settings. Physicians should be aware of the association between organizing pneumonia and the repetitive administration of rituximab. as well as any pulmonary event.
References
1. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B cell lymphoma. N Engl J Med. 2002; 346:235–242. PMID: 11807147.
2. Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005; 23:4117–4126. PMID: 15867204.

3. Sehn LH, Donaldson J, Filewich A, Fitzgerald C, Gill KK, Runzer N, et al. Rapid infusion rituximab in combination with corticosteroid containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood. 2007; 109:4171–4173. PMID: 17244675.
4. Mohrbacher A. B cell non-Hodgkin's lymphoma: rituximab safety experience. Arthritis Res Ther. 2005; 7(Suppl 3):S19–S25. PMID: 15960818.
5. Biehn SE, Kirk D, Rivera MP, Martinez AE, Khandani AH, Orlowski RZ. Bronchiolitis obliterans with organizing pneumonia after rituximab therapy for non-Hodgkin's lymphoma. Hematol Oncol. 2006; 24:234–237. PMID: 16948177.

6. Macartney C, Burke E, Elborn S, Magee N, Noone P, Gleadhill I, et al. Bronchiolitis obliterans organizing pneumonia in a patient with non-Hodgkin's lymphoma following R-CHOP and pegylated filgrastim. Leuk Lymphoma. 2005; 46:1523–1526. PMID: 16194900.

7. Leon RJ, Gonsalvo A, Salas R, Hidalgo NC. Rituximab-induced acute pulmonary fibrosis. Mayo Clin Proc. 2004; 79:949. 953. PMID: 15244399.

8. Mian M, Rass C, Hutarew G, Kofler B, Fiegl M, Greil R. Extensive organizing pneumonia during chemo-immunotherapy containing rituximab and G-CSF in a patient with diffuse large B-cell lymphoma: case report and review of the literature. Leuk Lymphoma. 2006; 47:1683–1685. PMID: 16966286.

9. Burton C, Kaczmarski R, Jan-Mohamed R. Interstitial pneumonitis related to rituximab therapy. N Engl J Med. 2003; 348:2690–2691. PMID: 12826649.

10. Brusamolino E, Rusconi C, Montalbetti L, Gargantini L, Uziel L, Pinotti G, et al. Dose-dense R-CHOP-14 supported by pegfilgrastim in patients with diffuse large B-cell lymphoma: a phase II study of feasibility and toxicity. Haematologica. 2006; 91:496–502. PMID: 16537117.
12. Astudillo L, Martin-Blondel G, Sans N, Dhaste G, Couret B, Arlet-Suau E. Solitary nodular form of bronchiolitis obliterans organizing pneumonia. Am J Med. 2004; 117:887–888. PMID: 15589500.

13. Epler GR. Bronchiolitis obliterans organizing pneumonia. Arch Intern Med. 2001; 161:158–164. PMID: 11176728.

14. Alexandrescu DT, Dutcher JP, O'Boyle K, Albulak M, Oiseth S, Wiernik PH. Fatal intra-alveolar hemorrhage after rituximab in a patient with non-Hodgkin lymphoma. Leuk Lymphoma. 2004; 45:2321–2325. PMID: 15512824.

15. Cordier JF, Loire R, Brune J. Idiopathic bronchiolitis obliterans organizing pneumonia. Definition of characteristic clinical profiles in a series of 16 patients. Chest. 1989; 96:999–1004. PMID: 2805873.
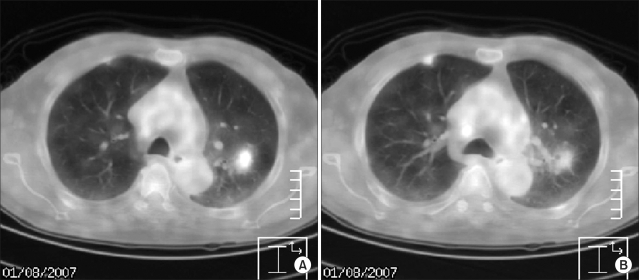
Fig. 1
Positron emission tomography images show focal hypermetabolic lesions in the apicoposterior segment of the left upper lobe (max SUV 2.2)(A) and in the anterior segment of the right upper lobe (max SUV 1.3)(B).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download