Abstract
Purpose
Retinoids have been shown to be effective in suppressing tumor development when chemical carcinogens such as N-nitroso-N-methylurea (NMU) and N-nitroso-N-ethylurea (NEU) were used to induce mammary tumors in a variety of animal models. However, the molecular mechanisms associated with the retinoid-mediated chemopreventive process, as linked to transcription factor NF-κB activation, for chemoprevention have not been elucidated. The purpose of this study was to determine the implications of NF-κB activation on the chemopreventive role of retinoids and their effect on cellular NF-κB activity that's induced by known alkylating chemical carcinogens such as NMU and NEU in human transfectant squamous cell carcinoma (SCC-13) cells.
Materials and Methods
The activity of NF-κB, as regulated by chemical carcinogens and retinoids, was determined in cultured human SCC-13 keratinocytes that were transfected with the pNF-κB-SEAP-NPT plasmid; this permitted the expression of the secretory alkaline phosphatase (SEAP) reporter gene in response to the NF-κB activity, and the plasmid contained the neomycin phosphotransferase (NPT) gene, which confers resistance to geneticin. The reporter enzyme activity was measured using a fluorescence detection assay method.
Results
All-trans retinoic acid and 13-cis retinoic acid induced a reduction of NF-κB activity up to 64% and 65%, respectively, compared to the control. For the treatment of the human transfectant cells with chemical carcinogens, all-trans retinoic acid (5 mM) and 13-cis retinoic acid (5 mM) downregulated the cellular NF-κB activation up to 83% and 85% compared to the NF-κB activity that was upregulated by NMU (5µM) and NEU (5µM), respectively.
Transcription factor NF-κB is expressed in all types of human cells and it influences the regulation of genes, including many of those involved in the inflammatory and immune response (1,2). NF-κB is found in the cytoplasm as an inactive form that is complexed with IκB. Stimulation of cells with a variety of environmental agents results in the rapid transit of cytoplasmic NF-κB to the nucleus. Several studies have demonstrated that the transcription factor NF-κB is involved in the development or progression of various human cancers, as well as in the regulation of apoptosis in both normal and cancer cells (3,4).
Retinoids are the natural and synthetic derivatives of vitamin A, and they have been shown to be important factors in the control of cell growth and differentiation, apoptosis and carcinogenesis (5~7). These effects are believed to result from interactions of the retinoids with the nuclear retinoid receptors such as the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs) (8,9). The binding of retinoids to their receptors is followed by the activation or inhibition of transcription of the retinoid-responsive genes. Retinoids have been shown to be effective in suppressing tumor development when chemical carcinogens such as N-nitroso-N-methylurea (NMU) and N-nitroso-N-ethylurea (NEU) are used to induce mammary tumors in a variety of animal models, and the retinoids are being studied for their potential to prevent cancer (10~12). However, the molecular mechanisms by which the retinoids mediate the chemopreventive process that's linked to transcription factor NF-κB activation have not been elucidated.
The activation of NF-κB in skin cells is considered to be a valuable target for therapeutic intervention; this is because skin is a primary target for a variety of environmental chemical carcinogens. Therefore, the ability of all-trans retinoic acid (tretinoin) and 13-cis retinoic acid (isotretinoin) to regulate the NF-κB activation that's induced by well known chemical carcinogens such as NMU and NEU in cultured human epidermal keratinocytes, squamous cell carcinoma (SCC) was examined (13).
This present study suggests the possible chemopreventive mechanism of retinoids, which involves the down-regulatory effects on NF-κB activation in human epidermal keratinocytes.
A Great EscAPe Fluorescence detection kit was obtained from Clontech Laboratories, Palo Alto, CA. The cell culture media and geneticin (antibiotic G-418) were purchased from Gibco BRL., Grand Island, NY. All-trans retinoic acid (vitamin A acid; tretinoin; t-RA), 13-cis retinoic acid (isotretinoin; c-RA), N-nitroso-N-methylurea (NMU) and N-nitroso-N-ethylurea (NEU) were obtained from Sigma Chemical Co., St. Louis, MO. The other chemicals and solvents were purchased from Aldrich Chemical Co., Milwaukee, WI. The human epidermal squamous cell carcinoma cell line SCC-13 (13), which was subcultured by this laboratory, was used for the experiments. The construction of the pNF-κB-SEAP-NPT plasmid (Fig. 1) has been previously described in detail (14). All the retinoids, NMU and NEU, were diluted with culture media to derive a stock solution and then they were directly added to the culture media.
Transfectant human SCC-13 cells were cultured with 500µg/ml of geneticin (100 mg/ml) for selecting and maintaining the stable transformants. The cell cultures were maintained at subconfluence in a 95% air and 5% CO2 humidified atmosphere at 37℃. The medium used for routine subcultivation was Dulbecco's Modified Eagle's Medium (Gibco, Grand Island, NY) that was supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/ml) and streptomycin (100µg/ml), and it was changed every 48~72 h as necessary. The cells were counted with a hemocytometer and the number of viable ells was determined by a trypan blue dye exclusion assay.
Transfection was essentially carried out as described previously for the preparation of the transfectant HaCaT cells (14). SCC-13 cells were plated at a density of 1.5×105 cells per well on six-well Costar plates (35 mm, Cambridge, MA) in 2 ml of media and then they incubated overnight. The cells were rinsed twice with serum-free media and next exposed with complexes containing pNF-κB-SEAP-NPT (6µg/100µl) and lipofectamine (25µg/100µl, Life Technologies) in 1 ml of serum-free media for 2 h. After the cells were transferred to complete medium and allowed to proliferate without selective pressure for another 48 h incubation, the transfected cells were maintained with geneticin for selection of the stably transfected cells.
The reporter enzyme (SEAP) was measured essentially as described previously with using a fluorescence detection assay method (14). The single cell-derived stable transfectant SCC-13 cells were plated on 5 ml of a T-25 flask. Upon reaching 30% confluency, the media was decanted. The cultures were washed twice with phosphate-buffered saline (PBS), and incubation was initiated with the addition of new media. The transfectant SCC-13 cells were treated with N-nitroso-N-methylurea and N-nitroso-N-ethylurea at concentrations of 5µM each in the culture medium. All the retinoids at concentrations of 1 and 5 mM were added to the culture media at 48 h of incubation. No treatment controls were included for each experiment. Aliquots (25µl) of medium for both the control and chemical-treated cultures were removed at 0, 24, 48 and 72 h, and these heated at 65℃ for 5 min to eliminate the endogenous alkaline phosphatase activity; they were then used immediately or they were stored at -20℃. Mixtures that contained dilution buffer (25µl), assay buffer (97µl), culture media (25µl), and 4-methylumbellifery phosphate (MUP, 1 mM, 3µl) in the 96 well plate were incubated for 60 min in the dark at room temperature. The fluorescence from the product of the SEAP/MUP was measured using a 96 well plate fluorometer (Molecular Devices, F max) by excitation at 360 nm and measurement of the light emission at 449 nm.
The cytotoxicity of the alkylating agents and retinoic acids was evaluated by the MTT assay (15). In brief, the transfectant SCC-13 (3×104) cells were plated on 96 wells of the ELISA plate, incubated for 48 h and then washed twice with 100µl of PBS. They were incubated with NMU (5µM) and NEU (5µM) for 48 h and then directly exposed to the retinoic acids (5 mM). After 24 h of incubation, extension of incubation for another 3 h at 37℃ was carried out with 200µl of MTT in PBS. Then, the solution was removed and formazan that was dissolved in 200µl dimethyl sulfoxide was added. The absorbance of the solution was measured at 540 nm using an ELISA plate reader. A control was provided without alkylating agents and retinoic acids in the medium.
The data is summarized as means±SDs. To statistically analyze the data, one-way analysis of variance (ANOVA) for repeated measurements of the same variable was performed. Duncan's multiple range t-test was then used to determine which means were significantly different from the mean of the control. A significant difference in the NF-κB activities between the carcinogen treated samples and retinoid treated samples was indicated by p<0.01.
For the quantitative measurement of the NF-κB activation induced by alkylating chemical carcinogens and retinoids, the human squamous cell carcinoma (SCC-13) cells that were transfected with the pNF-κB-SEAP-NPT plasmid (Fig. 1) were used. The transfectant SCC-13 cells released secretory alkaline phosphatase (SEAP) as a reporter gene and this contained the neomycin phosphotransferase (NPT) gene as a selection marker. Reporter enzyme (SEAP) activity can be simply measuredusing a fluorescence detection assay method by repeatedly sampling the cell culture (14). Therefore, this cell-based stable assay system is not needed to prepare the cell lysates for monitoring the NF-κB activity. The SCC-13 cells are a malignant epidermal keratinocyte line that's derived from a human squamous cell carcinoma; these cells can be cultured on a fairly routine basis and serve as a suitable model for aberrant terminal differentiation (13,16). Therefore, this cell line was selected to examine the influence of retinoids on NF-κB activation in keratinocytes. In this cell-based assay system for the quantitative measurement of the level of NF-κB activity, all-trans retinoic acid and 13-cis retinoic acid inhibited the cellular NF-κB activation up to 64% and 65%, respectively, compared to that of the control; the degree of inhibition increased in a dose-dependent manner (with using 1 and 5 mM of retinoids) (Fig. 2). Remarkably, the all-trans retinoic acid and 13-cis retinoic acid also showed significant downregulation of the cellular NF-κB activities that were upregulated by NMU (5µM) and NEU (5µM) in a dose-responsive manner (with using 1 and 5 mM of retinoids), respectively (Fig. 3, 4). The downregulation was up to 83~85% compared to the NMU-induced and NEU-induced cellular NF-κB activities in the cultured transfectant human keratinocytes (Fig. 3, 4). These results demonstrated that the degree of downregulation produced by the retinoic acids and with NMU and NEU treatment was higher than those produced in the absence of NMU and NEU. These results suggested that the NF-κB activity might have a role in the chemopreventive effect of the retinoic acids.
Retinoids have been shown to affect transcriptional regulation by inhibiting a number of transcription factors such as AP-1 and STAT-3 (17). The inhibition of AP-1 activity by retinoids in primary keratinocytes has been shown to provide a chemopreventive effect from the retinoids on ultraviolet B radiation-associated skin damage (18). In addition, retinoids, as regulators of gene transcription, have been shown to inhibit the phorbol ester (PMA)-induced MMP-1 and MMP-3 expression in human breast cancer cells (19). Moreover, they have been shown to suppress the PMA-mediated induction of cyclooxygenase-2 in human oral epithelial cells (20) and the iNOS gene expression in 1929 cells as well (21). Retinoids have been studied in several biological systems as potential chemopreventive agents and for the treatment of a variety of human cancers (22~24). It has also been reported that retinoids inhibit cell proliferation, modulate cell differentiation and enhance apoptosis. Mammary tumor formation in rodents, which was induced by chemical carcinogens, can be prevented by treatment with retinoids and their effects are mediated by retinoic acid receptors (25).
The chemopreventive role of retinoids is well established; however, the mechanisms underlying the chemopreventive effects remain unclear. Therefore, the primary intent of this study was to determine the possible involvement of cellular NF-κB activity in retinoid-modulated chemoprevention in an in vitro human skin cancer model. To test the hypothesis that the transcription factor NF-κB is involved in the chemopreventive role of retinoids, the effect of retinoid treatment on the NF-κB activation induced by NMU and NEU in squamous cell carcinoma cell line 13 (SCC-13) keratinocytes was investigated; the results were measured and compared with the samples treated under the same conditions, but in the absence of NMU and NEU.
It has been previously reported that the alkylating carcinogens NMU and NEU significantly upregulate cellular NF-κB activity. It has been suggested that the carcinogenicity resulting from alkylating chemicals might be associated with the modulation of cellular NF-κB activity in human skin cells (16). These findings led to examine whether a well known chemopreventive agent, such as the retinoids, could be used as a regulator of NF-κB activation when the cellular NF-κB activity is enhanced by chemical carcinogens. Thus, the ability of retinoids to regulate the cellular NF-κB activation, following treatment with the alkylating chemical carcinogens NMU and NEU, was evaluated to determine whether retinoids modulate the activation of NF-κB in cultured human keratinocytes. The results of this study demonstrated that treatment with retinoids resulted in significant downregulation of NMU induced and NEU induced cellular NF-κB activity; this suggests a possible role for NF-κB activity in the chemopreventive effects of retinoids as well as it being a possible mechanism for retinoid activity. Further studies are needed to elucidate the mechanistic details of the cellular NF-κB inactivation pathway that's associated with retinoids, and this might provide new insight on the prevention of cancer.
References
1. Baeuerle PA, Baichwal VR. NF-κB as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997; 65:111–137. PMID: 9238509.
2. Wulczyn FG, Krappmann D, Scheidereit C. The NF-κB/Rel and I kappa B gene families: mediators of immune response and inflammation. J Mol Med. 1996; 74:749–769. PMID: 8974017.
3. Bours V, Dejardin E, Goujon-Letawe F, Merville MP, Castronovo V. The NF-kappa B transcription factor and cancer: high expression of NF-kappa B-and I kappa B-related proteins in tumor cell lines. Biochem Pharmacol. 1994; 47:145–149. PMID: 8311838.
4. Bours V, Bentires-Alj M, Hellin AC, Viatour P, Robe P, Delhalle S, et al. Nuclear factor-κB, cancer, and apoptosis. Biochem Pharmacol. 2000; 60:1085–1089. PMID: 11007945.

5. Rudkin GH, Carlsen BT, Chung CY, Huang W, Ishida K, Anvar B, et al. Retinoids inhibit squamous cell carcinoma growth and intercellular communication. J Surg Res. 2002; 103:183–189. PMID: 11922733.

7. Lippman SM, Kessler JF, Meyskens FL Jr. Retinoids as preventive and therapeutic anticancer agents (Part I). Cancer Treat Rep. 1987; 71:391–405. PMID: 3548957.
8. Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987; 330:624–629. PMID: 2825036.

9. Benbrook D, Lernhardt E, Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988; 333:669–672. PMID: 2836738.

10. Zarbl H, Sukumar S, Arthur AV, Martin-Zanca D, Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-Nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. Nature. 1985; 315:382–385. PMID: 3923365.
11. Nakamura T, Ushijima T, Ishizaka Y, Nagao M, Nemoto T, Hara M, et al. Neu proto-oncogene mutation is specific for the neurofibromas in a N-nitroso-N-ethylurea-induced hamster neurofibromatosis model but not for hamster melanomas and human Schwann cell tumors. Cancer Res. 1994; 54:976–980. PMID: 7906199.
12. Likhachev AJ, Ivanov MN, Bresil H, Planche-Martel G, Montesano R, Margison GP. Carcinogenicity of single doses of N-nitroso-N-methylurea and N-nitroso-N-ethylurea in syrian golden hamsters and the persistence of alkylated purines in the DNA of various tissues. Cancer Res. 1983; 43:829–833. PMID: 6848195.
13. Rheinwald JG, Beckett MA. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell. 1980; 22:629–632. PMID: 6160916.

14. Moon KY, Hahn BS, Lee J, Kim YS. A cell-based assay system for monitoring NF-κB activity in human HaCaT transfectant cells. Anal Biochem. 2001; 292:17–21. PMID: 11319812.

15. Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988; 48:4827–4833. PMID: 3409223.
16. Moon KY, Lee YJ, Kim YS. Upregulation of cellular NF-κB activity by alkylating carcinogens in human epidermal keratinocytes. Biol Pharm Bull. 2003; 26:1195–1197. PMID: 12913277.
17. Paillaud E, Costa S, Fages C, Plassat JL, Rochette-Egly C, Monville C, et al. Retinoic acid increases proliferation rate of GL-15 glioma cells, involving activation of STAT-3 transcription factor. J Neurosci Res. 2002; 67:670–679. PMID: 11891779.

18. Darwiche N, Bazzi H, El-Touni L, Abou-Lteif G, Doueiri R, Hatoum A, et al. Regulation of ultraviolet B radiation-mediated activation of AP1 signaling by retinoids in primary keratinocytes. Radiat Res. 2005; 163:296–306. PMID: 15733037.

19. Dedieu S, Lefebvre P. Retinoids interfere with the AP1 signalling pathway in human breast cancer cells. Cell Signal. 2006; 18:889–898. PMID: 16176868.

20. Mestre JR, Subbaramaiah K, Sacks PG, Schantz SP, Tanabe T, Inoue H, et al. Retinoids suppress phorbol ester-mediated induction of cyclooxygenase-2. Cancer Res. 1997; 57:1081–1085. PMID: 9067275.
21. Oh GS, Pae HO, Seo WG, Shin MK, Kim IK, Chai KY, et al. Inhibitory effect of retinoic acid on expression of inducible nitric oxide synthase gene in 1929 cells. Immunopharmacol Immunotoxicol. 2001; 23:335–342. PMID: 11694025.
22. Moon RC, Mehta RG, Rao KVN. Sporn MB, Roberts AB, Goodman DS, editors. Retinoids and cancer in experimental animals. The Retinoids: biology, chemistry, and medicine. 1994. New York: Raven Press;p. 573–595.
23. Lippman SM, Meyskens FL Jr. Treatment of advanced squamous cell carcinoma of the skin with isotretinoin. Ann Intern Med. 1987; 107:499–502. PMID: 3477106.

24. Koh YM, Park JS, Namkoong SE, Lee SY, Kim SA, Hong KJ, et al. Growth regulation of ovarian cancer cells through the inactivation of AP-1 by retinoid derivatives. J Korean Cancer Assoc. 2000; 32:1043–1049.
25. Okuno M, Kojima S, Matsushima-Nishiwaki R, Tsurumi H, Muto Y, Friedman SL, et al. Retinoids in cancer chemoprevention. Curr Cancer Drug Targets. 2004; 4:285–298. PMID: 15134535.

Fig. 1
Diagram of the pNF-κB-SEAP-NPT plasmid. The plasmid permits expression of the secretory alkaline phosphatase (SEAP) reporter gene in response to the NF-κB activity and it contains the neomycin phosphotransferase (NPT) gene for the geneticin resistance in host cells.
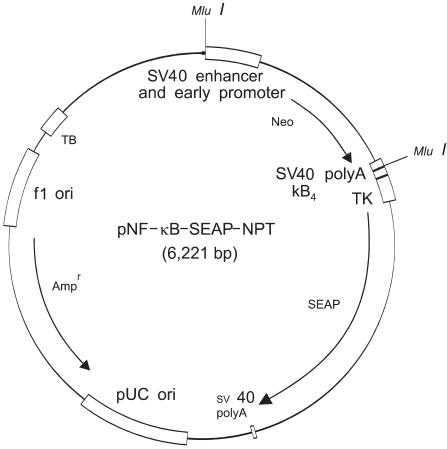
Fig. 2
Dose-dependent downregulation by retinoic acid on the cellular NF-κB activity in human transfectant SCC-13 cells. Panel (A), All-trans retinoic acid effect on the cellular NF-κB activity; Panel (B), 13-cis retinoic acid effect on the cellular NF-κB activity. Retinoids were added to the culture medium after 48 h of incubation. The SEAP activities were measured 24 h after exposure to chemicals. Each value represents the mean±SD of three determinations. RLU stands for relative light units. A significant difference in the NF-κB activities between retinoid treated cells and the control is indicated by *p<0.01.
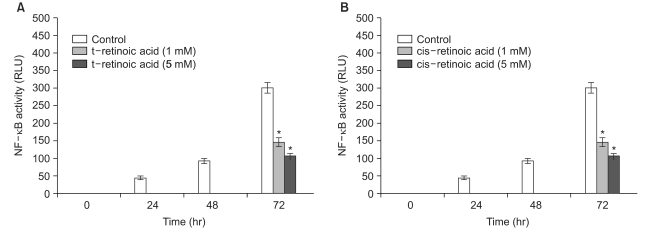
Fig. 3
Dose-dependent downregulation by All-trans retinoic acid on the cellular NF-κB activity induced by NMU and NEU in human transfectant SCC-13 cells. Panel (A), All-trans retinoic acid effect on the NMU-upregulated cellular NF-κB activity; Panel (B), All-trans retinoic acid effect on the NEU-upregulated cellular NF-κB activity. A significant difference in the NF-κB activities between the control and alkylating carcinogen treated cells is indicated by *p<0.01. A significant difference in the NF-κB activities between alkylating carcinogen treated cells and the all-trans retinoic acid treated cells is indicated by †p<0.01.
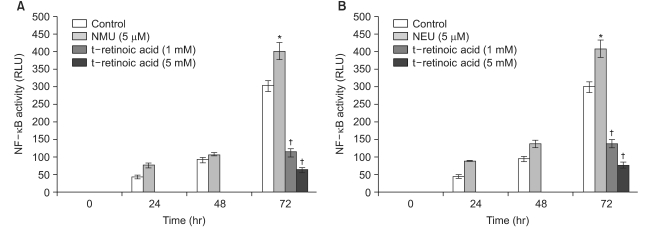
Fig. 4
Dose-dependent downregulation by 13-cis retinoic acid on the cellular NF-κB activity induced by NMU and NEU in human transfectant SCC-13 cells. Panel (A), 13-cis retinoic acid effect on the NMU-upregulated cellular NF-κB activity; Panel B, 13-cis retinoic acid effect on the NEU-upregulated cellular NF-κB activity. A significant difference in the NF-κB activities between the control and alkylating carcinogen treated cells is indicated by *p<0.01. A significant difference in the NF-κB activities between the alkylating carcinogen treated cells and the 13-cis retinoic acid treated cells is indicated by †p<0.01. Alkylating chemicals and retinoids were added to the culture medium at 0 and 48 h of incubations, respectively. The SEAP activities were measured at 24, 48 and 72 h after exposure to the chemicals. Each value represents the mean±SD of three determinations. RLU stands for relative light units.
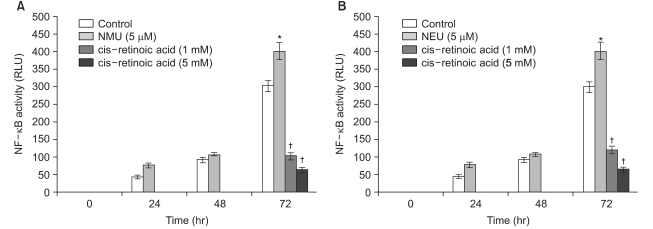
Table 1
Relative mitochondrial dehydrogenase (MDH) activity from the alkylating agents and retinoic acids in human transfectant SCC-13 cells
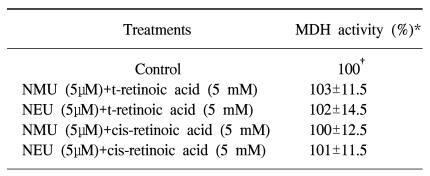
*Mean±SD of three experiments. †This value is defined as 100 for each experiment. Cell survival was determined by measuring the MDH activities of the transfectant SCC-13 cells when they were exposed to the concentrations of the alkylating agents and retinoids tested. No significant difference in cell viability was found between the chemical-treated and nontreated cells.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download