Abstract
Purpose
To evaluate the therapeutic activity and safety of paclitaxel and cisplatin combination chemotherapy in patients with advanced or metastatic gastric cancers that are unresponsive to primary chemotherapy.
Materials and Methods
Advanced or metastatic gastric cancer patients unresponsive to first line chemotherapy were entered into this trial. The treatment regimen consisted of paclitaxel, 175 mg/m2 by 3-hour infusion on day 1, and cisplatin, 60 mg/m2 by 1 hour infusion on day 1, with the treatment repeated every 3 weeks.
Results
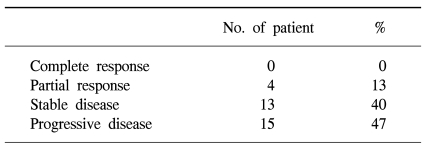
37 patients were entered in this study, with 32 fully evaluable for response. 4 (13%), 13 (40%) and 15 (47%) patients achieved a partial response, stable disease and progressed, respectively. The median time to progression was 4.0 months (95% CI: 2.0~6.0 months), and the median overall survival was 12.6 months (95% CI: 5.5~19.7 months), with a 1-year survival rate of 54%. Of a total of 135 cycles of chemotherapy, grades 3 and 4 hematological toxicities were neutropenia (14%) and anemia (3%). Grade ≥2 neuropathy was observed in 6 patients (17%).
Gastric cancer is the most common malignancy in Korea (1), and the leading cause of death due to cancer worldwide (2) with the exception of only lung cancer. Despite the development of early gastric cancer detection programs, only about 30% of patients are operable on presentation, and the incidence of relapse is high. In general, the prognosis is poor, and the 5 year overall survival of patients with advanced gastric cancer is only 5 to 15% (3).
In general, 5-fluorouracil (5-FU)-based or cisplatin-based combination regimens are widely accepted as potential standard therapies, with response rates of around 25~40% and median overall survival of 7~9 months (4). Another study has shown that the combination of epirubicin, cisplatin and infusional 5-FU (ECF) was highly effective as a first-line treatment for advanced gastric cancer (5). However, most cases of locally advanced or metastatic gastric carcinomas ultimately progress and eventually result in patient death, regardless of the patient's initial response to chemotherapy. For these patients, there are still no standard palliative chemotherapy regimens or other treatment options currently available. Therefore, the need for highly active new agents and novel combinations of these drugs is imperative.
Paclitaxel is one of the most active anticancer drugs for the treatment of solid tumors, which effectively blocks cancer cells in the G2/M phase via the inhibition of microtubular depolymerization (6,7). An administration schedule of 175~225 mg/m2 intravenous infusion doses every 3 weeks has been widely accepted (8). In addition, several phase II studies have shown that paclitaxel, either alone or in combination with cisplatin or 5-fluorouracil, is also active against advanced gastric cancer (9~12). Paclitaxel appears to have a schedule- dependent synergy with platinum compounds, as documented in established human gastric cell lines (13).
This phase II study was designed to assess the safety and efficacy of a combination of paclitaxel and cisplatin chemotherapy as a salvage treatment for patients with advanced or metastatic gastric cancer.
Patients with metastatic or recurrent gastric adenocarcinomas, who had failed (primary or secondary after initial response) 5-FU-based chemotherapy within 1 year before their registration, were enrolled in this study. The patients were required to have a measurable disease, a life expectancy of 8 weeks or more, an ECOG performance status of 2 or less, adequate bone marrow (neutrophil count >1,000/mm3, and a platelet count >100,000/ mm3), hepatic (serum total bilirubin level <1.5 mg/dl, AST/ALT <3X ULN) and renal function (serum creatinine level <1.5 mg/dl). This trial was designed to detect a response rate of 30% compared with a minimal, clinically meaningful response rate of 10%, with a statistical power of 80% for accepting the hypothesis and 5% significance for rejecting the hypothesis. The total sample size required to allow for a follow-up loss rate of up to 10% was 35 patients with a measurable disease; all patients gave their written informed consent.
The treatment consisted of paclitaxel, 175 mg/m2, in a 3-hour infusion followed by cisplatin, 60 mg/m2, in a 1-hour infusion. Each cycle of chemotherapy was given every 3 weeks if the patient's blood count had returned to normal and non-hematological toxic effects had resolved. The dosage of the subsequent cycle was adjusted according to the toxic effects that had developed during the preceding cycle. All patients received a standard supportive regimen, consisting of hydration with normal saline at least 3 L/24 hours, dexamethasone and 5-HT3 inhibitors. Follow-up history and physical examinations, tumor measurements and toxicity assessments were performed before each 3-week cycle of therapy.
The tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (14), and was assessed by a computed tomographic scan of the abdomen and pelvis, and by the same tests initially used to stage the tumor. All tumor measurements were recorded by radiologists, in millimeters, for all measurable lesions. Responses were assessed after 2 and 4 courses of chemotherapy. Toxicity grading was based on the National Cancer Institute common toxicity criteria (NCI-CTC version 3.0). Patients were evaluated for toxic effects before each new treatment cycle. The survival duration was computed as the time between the first day of the second-line chemotherapy given as the protocol and the patient's death or, in the case of continuing survival, the last day of follow-up. The overall and progression-free survival curves were computed using the Kaplan-Meier method.
A total of 37 patients were registered between Feb. 2002 and Sep. 2006. The characteristics of the eligible patients are listed in Table 1. The male to female ratio was 21 : 16. The median patient age was 56 years (range: 28~79), and 73% of the patients had an Eastern Cooperative Oncology Group performance status of 0 or 1. Prior chemotherapy consisted of 5-fluorouracil- based or cisplatin-based regimens in almost all patients. Fifteen patients (40%) had previously undergone chemotherapy with more than 2 regimens. Eighteen patients had at least two organs involved. The majority of the patients had metastatic lymph nodes (51%), and 30% had liver metastases.
Of the 37 patients assessable for survival, 5 were not "evaluable" for response. Three patients had refused treatment prior to evaluation and 2 were lost to follow-up. Thus, 32 patients were assessable for response. 4 partial responses were obtained, corresponding to 13% of the evaluable patients. There were 13 patients with a stable disease (40%) and 15 progressed (47%) (Table 2). The median follow-up duration was 37 months. The median progression-free survival was 4 months (95% CI : 2~6 months) (Fig. 1). The median overall survival was 12.6 months (95% CI : 5.5~19.7 months), with a 1-year survival rate of 54% (Fig. 2).
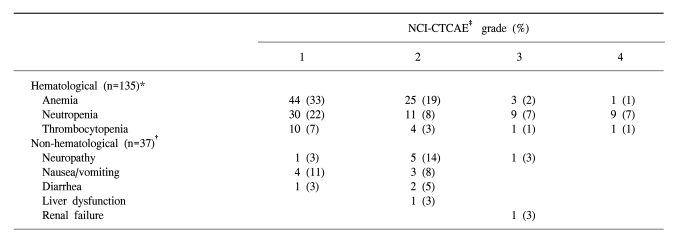
Thirty-seven patients received a total of 135 treatment cycles, and received a median of 3 courses of paclitaxel plus cisplatin (range, 1~9 courses). The planned dose intensities of paclitaxel and cisplatin were 58.3 and 20 mg/m2/week respectively. The relative dose intensities (the actual dose delivered as a proportion of the planned dose) of paclitaxel and cisplatin were 88 and 93%, respectively. The toxicity was evaluable in all 37 patients and for all 135 chemotherapy cycles. The most frequent hematological toxicity was anemia (grade 1 or 2 in 52% of treatment cycles). Grades 3 or 4 neutropenia were observed in 18 cycles (14%), but only 6 episodes of nonfatal neutropenic fever were observed. Grade I or II nausea/vomiting was observed in 7 patients (19%), and grade II neuropathy was reported in 5 patients (14%). One patient experienced an anaphylactic reaction, but there were no treatment-related deaths (Table 3).
Taxanes (paclitaxel and docetaxel) have been tried as single agents in the treatment of advanced gastric cancer. Paclitaxel was well tolerated, with reported overall response rates ranging between 17 and 23% (9,15,16).
The combination of paclitaxel and 5-FU, as a first line treatment with advanced gastric cancer, has shown a response rate of 65%, with a median survival of 12 months in 31 patients (17). Promising results have also been reported with a regimen of paclitaxel and cisplatin (18), with an overall response rate of 44%, with a median time to progression and an overall survival of 7 and 11.2 months, respectively.
In our study, each agent was used at initial doses of paclitaxel, 175 mg/m2, and cisplatin, 60 mg/m2, every 3 weeks, as it was believed that the safety and tolerability of the treatment were indispensable in the salvage setting for the treatment of solid tumors.
Using this dose schedule, an overall response rate of 13% and a median overall survival of 12.6 months were achieved. The reported second line treatment response rates have been variable, ranging between 10~40% (19~24). The reason for the lower response rate might be that 40% of all the patients had undergone more than a third-line salvage treatment.
Although the response rate was slightly low, the survival time was comparable with previous studies that had used weekly paclitaxel or other agents, including irinotecan, oxaliplatin and docetaxel, as a second-line treatment in patients with pretreated advanced gastric cancer (19~24). A performance status of 0 or 1 was reported in 73% of patients, and non-visceral organ metastasis-lymph node or bone occurred in a large portion (64%) of patients who entered our phase II study.
Previous reports have shown that grades 3 or 4 neutropenia occurred in 37% of patients, and grade 4 neutropenia in 67% of patients treated with paclitaxel with an every-3-weeks regimen (16). In our study, 14% of patients suffered severe neutropenia.
The relative dose intensities of paclitaxel and cisplatin were 88 and 93% of the planned doses, respectively. Grade 3 or 4 neutropenia occurred in 14% of the 135 treatment cycles. No deaths resulting from toxicity were observed.
References
1. Bae JM, Won YJ, Jung KW, Park JG. Annual report of the Korea Central Cancer Registry Program 2000: based on registered data from 131 hospitals. Cancer Res Treat. 2002; 34:77–83.

2. International Agency for Research on Cancer (IARC). 2000.
3. Ho D. Ho D, editor. Epidemiologic studies in gastric cancer. Gastric cancer. 1988. New York, NY: Churchill Livingstone;p. 1–25.
4. Wilke HJ, Van Cutsem E. Current treatments and future perspectives in colorectal and gastric cancer. Ann Oncol. 2003; 14(Suppl 2):ii49–ii55. PMID: 12810459.

5. Waters JS, Norman A, Cunningham D, Scarffe JH, Webb A, Harper P, et al. Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: results of a randomized trial. Br J Cancer. 1999; 80:269–272. PMID: 10390007.

6. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979; 277:665–667. PMID: 423966.

7. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996; 56:816–825. PMID: 8631019.
8. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–98. PMID: 11784875.

9. Ajani JA, Fairweather J, Dumas P, Patt YZ, Pazdur R, Mansfield PF. Phase II study of Taxol in patients with advanced gastric carcinoma. Cancer J Sci Am. 1998; 4:269–274. PMID: 9689986.
10. Bokemeyer C, Lampe CS, Clemens MR, Hartmann JT, Quietzsch D, Forkmann L, et al. A phase II trial of paclitaxel and weekly 24 h infusion of 5-fluorouracil/folinic acid in patients with advanced gastric cancer. Anticancer Drugs. 1997; 8:396–399. PMID: 9180395.

11. Kollmannsberger C, Quietzsch D, Haag C, Lingenfelser T, Schroeder M, Hartmann JT, et al. A phase II study of paclitaxel, weekly, 24-hour continuous infusion 5-fluorouracil, folinic acid and cisplatin in patients with advanced gastric cancer. Br J Cancer. 2000; 83:458–462. PMID: 10945491.

12. Kim MK, Lee KH, Hyun MS, Do YR, Song HS, Lee WS, et al. A multi-center, phase II clinical trial of Padexol (paclitaxel) and cisplatin for patients suffering with advanced gastric cancer. Cancer Res Treat. 2005; 37:349–353.

13. Vanhoefer U, Harstrick A, Wilke H, Schleucher N, Walles H, Schroder J, et al. Schedule-dependent antagonism of paclitaxel and cisplatin in human gastric and ovarian carcinoma cell lines in vitro. Eur J Cancer. 1995; 31A:92–97. PMID: 7695986.

14. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States. National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.

15. Cascinu S, Graziano F, Cardarelli N, Marcellini M, Giordani P, Menichetti ET, et al. Phase II study of paclitaxel in pretreated advanced gastric cancer. Anticancer Drugs. 1998; 9:307–310. PMID: 9635920.

16. Ohtsu A, Boku N, Tamura F, Muro K, Shimada Y, Saigenji K, et al. An early phase II study of a 3-hour infusion of paclitaxel for advanced gastric cancer. Am J Clin Oncol. 1998; 21:416–419. PMID: 9708646.

17. Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol. 1999; 22:580–586. PMID: 10597742.
18. Kornek GV, Raderer M, Schull B, Fiebiger W, Gedlicka C, Lenauer A, et al. Effective combination chemotherapy with paclitaxel and cisplatin with or without human granulocyte colony-stimulating factor and/or erythropoietin in patients with advanced gastric cancer. Br J Cancer. 2002; 86:1858–1863. PMID: 12085176.

19. Ajani JA, Baker J, Pisters PW, Ho L, Mansfield PF, Feig BW, et al. Irinotecan/cisplatin in advanced, treated gastric or gastroesophageal junction carcinoma. Oncology. 2002; 16(5):Suppl 5. 16–18. PMID: 12109800.
20. Suh SH, Kwon HC, Jo JH, Cho YR, Seo BG, Lee DM, et al. Oxaliplatin with biweekly low dose leucovorin and bolus and continuous infusion of 5-fluorouracil (Modified FOLFOX-4) as a salvage therapy for patients with advanced gastric cancer. Cancer Res Treat. 2005; 37:279–283.
21. Assersohn L, Brown G, Cunningham D, Ward C, Oates J, Waters JS, et al. Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol. 2004; 15:64–69. PMID: 14679122.

22. Chun JH, Kim HK, Lee JS, Choi JY, Lee HG, Yoon SM, et al. Weekly irinotecan in patients with metastatic gastric cancer failing cisplatin-based chemotherapy. Jpn J Clin Oncol. 2004; 34:8–13. PMID: 15020657.

23. Stathopoulos GP, Rigatos SK, Fountzilas G, Polyzos A, Stathopoulos JG. Paclitaxel and carboplatin in pretreated advanced gastric cancer: a phase II study. Oncol Rep. 2002; 9:89–92. PMID: 11748462.

24. Roth AD, Ajani J. Docetaxel-based chemotherapy in the treatment of gastric cancer. Ann Oncol. 2003; 14(Suppl 2):ii41–ii44. PMID: 12810457.

Fig. 1
Progression free survival of patients. The median progression-free survival was 4 months (95% CI: 2~6 months).
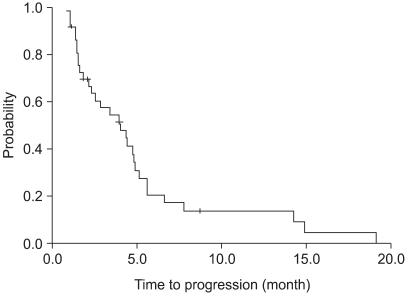
Fig. 2
Overall survival of patients. The median overall survival was 12.6 months (95% CI: 5.5~19.7 months) with a 1-year survival rate of 54%.
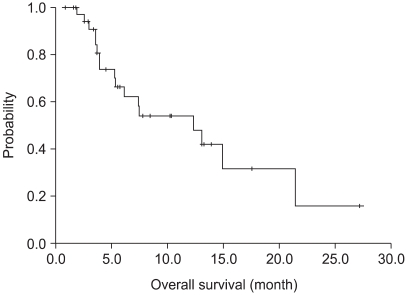
Table 1
Patient characteristics (n=37)
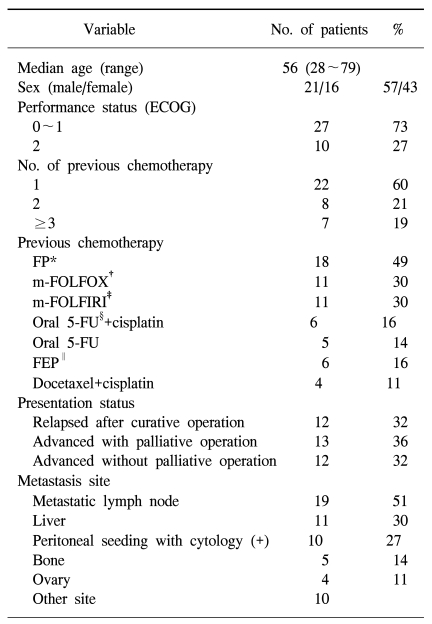
ECOG: Eastern Cooperative Oncology Group, *FP: 5-fluorouracil (5-FU)+cisplatin, †m-FOLFOX (modified-FOLFOX): oxaliplatin, low dose leucovorin, 5-FU bolus and continuous infusion every 14 days, ‡m-FOLFIRI (modified-FOLFIRI): irinotecan, low dose leucovorin, 5-FU bolus and continuous infusion every 14 days, §Oral 5-FU: capecitabine, TS-1 or UFT§, ∥FEP: 5-FU, etoposide, cisplatine.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download