Abstract
Purpose
High-dose chemotherapy (HDT) and autologous stem cell transplantation (ASCT) have been used for the treatment of clinically aggressive non-Hodgkin's lymphoma (NHL). However, the superiority of specific conditioning regimens has not yet been established. The present study evaluated the efficacy and toxicity of a conditioning regimen involving fractionated total body irradiation (TBI), and the use of Ara-C and melphalan (TAM) for clinically aggressive NHL.
Materials and Methods
Between March 2002 and December 2004, 31 patients with aggressive NHL received fractionated TBI with a dose of 12 Gy over 3 days, and were administered 9 g/m2 Ara-C and 100 mg/m2 melphalan followed by autologous peripheral blood stem Cell Transplantation at the Catholic Hematopoietic Stem cell transplantation Center Korea. Patients that responded to first line chemotherapy and achieved complete remission (CR), or were in a first sensitive relapse were defined as having less advanced disease, while the other patients were defined as having more advanced disease.
Results
Objective responses were obtained in 24 of 31 patients (77.4%), comprising complete remission in 19 patients (61.3%) and partial remission in 5 (16.1%) patients. The median follow-up time was 28 months (range 1~62 months). At 3 years, the overall survival and event-free survival (EFS) rates were 62.3% and 47.3%, respectively. Patients with less advanced disease and more advanced disease showed 3-year EFS rates of 73.3% and 22.5 %, respectively (p=0.006). Early (within the first 100 days) treatment-related mortality occurred in 3 (9.7%) patients. Of the 31 total patients, 15 (48.4%) developed grade 3 mucositis, 22 (70.9%) developed neutropenic fever, and two (6.5%) developed interstitial pneumonia syndrome>grade 3.
The rate of complete remission for disseminated, intermediate- and high-grade non-Hodgkin's lymphoma (NHL) is approximately 60~80% after standard treatment; however, the rate of long-term disease-free survival is only 30~50% (1). In particular, long-term survival is only 5~10% after salvage therapy in patients that do not attain remission or relapse after complete remission (1). Thus, a combination of high-dose therapy (HDT) and autologous stem cell transplantation (ASCT) has been used in patients with first or subsequent chemotherapy sensitive relapse, as well as in patients that fail induction chemotherapy (2,3). The efficacy of HDT with ASCT has also been reported in patients with a high risk of recurrence, even in remission (4).
Various preparative HDT regimens using chemotherapy only or in combination with total-body irradiation (TBI) have been explored, but none of the regimens has been found to be clearly superior (5,6). The previous reported TBI-based regimens mostly involved the use of cyclophosphamide alone or the use of cyclophosphamide and etoposide or the use of cyclopho sphamide and cytarabine (7~10). These studies showed that transplantation-related mortality and relapse remained major problems (7~9). A conditioning regimen comprising a combination of TBI, cytarabine and melphalan (TAM) was proposed for use prior to allogenic and autologous bone marrow transplantation for high risk acute lymphoblastic leukemia (10, 11). Cytosine arabinoside and melphalan are active drugs for the treatment of hematological malignancies and these two drugs combined with carmustine and etoposide are commonly used in a chemotherapy preparative regimen for NHL as the BEAM regimen (12,13). Our center previously reported the feasibility of a modified TAM regimen for autologous stem cell transplantation for acute myeloid leukemia patients in first complete remission (14). Based on the experience for acute leukemia and the recent report of our center in the experience of the use of the modified TAM conditioning regimen for AML, we have investigated the feasibility of the use of the TAM conditioning regimen for NHL. This conditioning regimen has not been evaluated in adult patients with NHL.
The current report describes the first single center study on the use of TAM as a conditioning regimen for NHL patients. The report provides information regarding the toxicity and efficacy of this regimen.
Between March 2002 and December 2004, 36 patients with histologically- proven NHL received TAM high dose therapy and autologous peripheral blood stem cell transplantation (APBSCT) at the Catholic Hematopoietic Stem cell transplantation Center of Korea. Patients were selected for this study based on the following eligibility criteria. The criteria included an age between 15 and 65 years, a confirmed histological diagnosis of aggressive NHL according to WHO classifications (15), a performance status at transplantation that should be ≤2 according to the ECOG (Eastern Cooperative Oncology Group) scale, adequate renal, hepatic, respiratory and cardiac functions; and the absence of active infection. Additional criteria included that the disease status at transplantation was one of the three following conditions: 1) patients that had relapsed but were chemotherapy-sensitive disease status, 2) patients that did not respond to first-line chemotherapy (stable disease or progressive disease) but achieved either complete remission (CR) or partial remission (PR) after salvage chemotherapy, 3) patients in first remission (CR or PR) with a high risk relapse based on high-intermediate (2 risk factors) or high ( 3 risk factors) risk of an age-adjusted International Prognostic Index (IPI) at the time of diagnosis (4)-these risk factors were lactate dehydrogenase (LDH) levels greater than one time normal; Ann Arbor stage III/IV and ECOG performance status at diagnosis 2 through 4 (16). All patients gave written informed consent before treatment. Of the 36 patients, 31 patients fulfilled the eligibility criteria, and were enrolled in the study.
Based on the chemotherapy sensitivity and the remission state prior to HDT, patients who respond to first line chemotherapy and achieved a CR or chemotherapy sensitive first relapse were classified as having less advanced disease (7). More advanced disease included 1) patients that did not respond to first-line chemotherapy or initially responded but progressed during salvage therapy and achieved remission after the last salvage chemotherapy, 2) patients that achieved PR during first-line chemotherapy but never achieved CR in 2 or more regimens (7). Patients initially diagnosed with primary central nervous system lymphoma were excluded.
Overall, 31 patients received peripheral blood stem cells (PBSC), and only three patients required additional bone marrow stem cells due to a low PBSC dose. PBSC were mobilized by the administration of chemotherapy and recombinant human granulocyte colony stimulation factor (rhG-CSF). Seventeen patients received the IVAM (ifosfamide 1,500 mg/m2 daily for 5 days plus mesna, etoposide 150 mg/m2 daily for 3 days, cytarabine 100 mg/m2 daily for 3 days and methotrexate 3 g/m2 on day 5 with leucovorin rescue) regimen for PBSC mobilization. Other chemotherapy regimens used were DHAP (dexamethasone 40 mg daily for 4 days, cisplatin 100 mg/m2 as a 24-hour continuous infusion day 1 and cytarabine 2 g/m2 q 12 hours on day 2; n=5) and mini BCNU, etoposide, cytarabine and melphalan (n=3). Patients received 5 µg/kg/day rhG-CSF from the fifth day after chemotherapy until a WBC count >3,000/µL or a CD 34 cell proportion >0.3 % was reached. The goal was to collect >2×106 CD34 cells/kg. Cryopreservation in liquid nitrogen, thawing and transfusion were performed according to standard procedures.
Thirty-one patients received the modified TAM regimen comprising TBI (12 Gy as 2 Gy fractions twice a day for 3 days) from days 6 to 8, followed by an intermediate dose of Ara-C (6 doses of 1.5 g/m2 over 3 hours every 12 hours for a total of 9 g) from days 3 to 5, and then melphalan (100 mg/m2 over 30 min) on day 2.
Prophylactic antibiotic therapy (ciprofloxacin, fluconazole) for gut decontamination was orally administered until the absolute neutrophil count (ANC) was >1.0×109/L for three consecutive days. All blood components were irradiated and leukocyte-filtered before transfusion. Autologous peripheral stem cells were infused on day 0. All patients received rhG-CSF from day +3 after PBSC infusion with a dose of 5 µg/kg/day subcutaneously until the ANC was >1.0×109/L.
All patients were evaluated for response 3 months after the day of stem cell infusion. Response was assessed according to the International Working Group response criteria (17). Further follow-up examinations were performed at 3-month intervals for the first year, and thereafter at 6-month intervals for at least 3 years, including tests that were abnormal prior to ASCT. After ASCT, engraftment was confirmed as an ANC >0.5×109/L and a platelet count >20×109/L with no platelet transfusion for 3 consecutive days. Toxicity was evaluated according to the National Cancer Institute-Common Toxicity Criteria (NCI-CTC). In terms of regimen-related toxicities; interstitial pneumonia syndrome (IPS) was defined as diffuse pulmonary infiltrates without an identifiable infectious cause. Venoocclusive disease (VOD) of the liver was defined as liver toxicity secondary to chemotherapy or TBI, and the diagnosis was made on the basis of hepatomegaly and/or liver tenderness, a weight gain >2.5% of the baseline and an elevated serum bilirubin level >2 mg/dl. A diagnosis was also made with or without histological evidence of endothelial cell damage at the terminal hepatic venules, dilatation of the sinusoids and necrosis of hepatocytes (18).
Event-free survival (EFS) was defined as the period from the time of stem cell infusion to relapse, progress or death from any cause or censored at the last follow-up date. Overall survival was defined as the period from the time of stem cell infusion to the time of death or censored at the last follow-up date. OS and EFS were calculated using the Kaplan-Meier method and compared by log-rank test. The Cox regression analysis was performed for multivariate analysis to identify prognostic factors associated with OS and EFS. The following factors were initially planed to be examined: age (≤60 vs >60 years), sex, histology, LDH (≤1 vs >1 normal value), disease stage (I~II vs III~IV), performance status at diagnosis (ECOG 0,1 vs ≥2), number of extranodal sites (<1 vs ≥2), bone marrow involvement, CSF involvement, tumor size (<10 cm vs ≥10 cm), age-adjusted IPI score (0,1 vs ≥2), number of previous chemotherapy regimens (1,2 vs ≥3) and status of the disease before transplantation (less advanced disease vs more advanced disease). However, no patient was older than 60 years or had CSF involvement; age and CSF involvement factors were excluded. All analyses were based on a retrospective review. Statistical analysis was performed using SPSS software version 11.0 (SPSS Inc., Chicago, IL, USA).
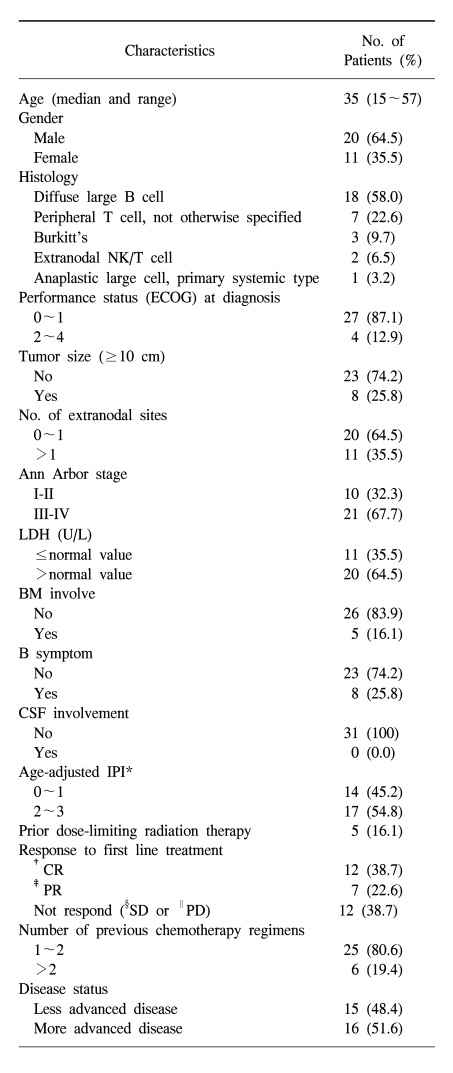
Patient characteristics at the time of ASCT are summarized in Table 1. Thirty-one NHL patients between the ages of 15 and 57 years (median 37 years) underwent a TAM conditioning regimen following APBSCT. The patient population consisted of 20 males and 11 females. In terms of histological subtypes, there were 18 diffuse large B-cell (DLBCL) subtypes, 7 peripheral T cell (PTCL) subtypes, 3 Burkitt's subtypes, 2 extranodal NK/T cell subtypes and 1 primary systemic anaplastic large cell subtype. The combination of cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) was the most common regimen used for first-line chemotherapy (n=25). Other chemotherapy regimens used were ProMACE-CytaBOM (n=1), COPBLAM (n=1) and CODOX-M/IVAC (n=3) for Burkitt's lymphoma. No patient was treated with rituximab-based chemotherapy. Response to the first-line chemotherapy was as follows: CR in 12 patients (38.7%), PR in 7 patients (22.6%) and stable disease or progressed disease in 12 patients (38.7%). The most common salvage regimen was IVAM (n=21). Other salvage chemotherapy regimens were DHAP (n=4) and MACOP-B (n=2). The median number of regimens was 2 (range 1~4) and the median number of cycles was 8 (range 4~13). Two regimens were used in 23 (74.2%) patients, 3 regimens in 3 patients and 4 regimens in 3 patients. Only two patients with Burkitt's lymphoma were treated with one regimen (CODOX-M/IVAC).
For 18 patients with DLBCL subtypes, 8 patients did not respond to first-line chemotherapy; 5 patients had a high risk relapse and 5 had a sensitive relapse. In 8 patients that did not respond to first-line chemotherapy with DLBCL subtypes, 6 patients had an age-adjusted IPI score ≥2. For 7 patients with PTCL subtypes, all of the patients had an age-adjusted IPI score ≥2 and 3 of the patients did not respond to first line chemotherapy. For 3 patients with Burkitt's lymphoma, all three patients had an age-adjusted IPI score ≥2 and one patient did not respond to first line chemotherapy. For 2 patients with NK/T cell lymphoma, one patient had a sensitive relapse and the other patient had high risk relapse disease status. One patient with anaplastic lymphoma did not respond to first line chemotherapy. For 8 patients (25.8%) with a bulky mass, 3 patients had Burkitt's lymphoma and 5 patients had DLBCL subtypes. Among the 5 patients with DLBCL subtypes, 4 patients were Ann Arbor stage IV, one was stage III and all of the patients had an age-adjusted IPI sore ≥2. Six of 8 patients with a bulky mass had a high-risk disease status and 2 patients did not respond to first-line chemotherapy. One of 8 patients received involved field radiation before transplantation.
In terms of pretransplantation disease status, 15 patients had less advanced disease and 16 patients had more advanced disease. For less advanced disease, 9 patients (29%) achieved CR with high-risk disease and 6 patients (12.9%) had a first sensitive relapse. In these first sensitive relapsed patients, CHOP was the most common regimen used for first-line chemotherapy (n=5), followed by the use of the ProMACE-CytaBOM regimen (n=1). The salvage regimens in these patients were IVAM (n=3) and DHAP (n=3). In the more advanced disease group of patients, 13 (41.9%) of the 16 patients did not respond to first-line chemotherapy; 3 (9.7%) patients achieved PR after first-line chemotherapy but never achieved CR after treatment with two or more chemotherapy regimens. Among the 13 patients that did not respond to first-line chemotherapy, 2 patients achieved CR after third-line chemotherapy and 11 patients achieved PR after a final treatment with salvage chemotherapy. For the more advanced disease group of patients, the median number of regimens was 3 (range 2~4) and the median number of cycles was 8 (range 5~13). Five patients received prior involved-field radiation. Two of them patients received palliative radiation. Three of the patients had localized disease and received radiation with a curative intent. For these three patients, two patients progressed during first-line chemotherapy and did not respond to radiotherapy, and the last patient relapsed 8 months after first-line chemotherapy and radiotherapy. The radiation dose to the tumor bed was between 36 and 45 Gy, with a median dose of 40 Gy.
One patient died on day 6 and another died on day 43 prior to engraftment because of multiorgan failure associated with clinical sepsis syndrome. The median number of CD34+ cells infused was 4.72×106/kg (range; 2.06~46.95×106/kg), and the median number of mononuclear cells infused was 3.98×108/kg (range: 0.5~12.50×108/kg). Granulocyte recovery to >0.5×109/l took a median of 11 days (range 5~17 days), and platelet recovery to >20×109/l without support for 3 consecutive days took a median of 13 days (range 7~38 days). The median number of packed red cell transfusions was 6 units (range: 0~14), and the median number of platelet transfusions was 7 units (range 1~55). The median time to discharge following stem cell infusion was 19 days (range 11~39 days).
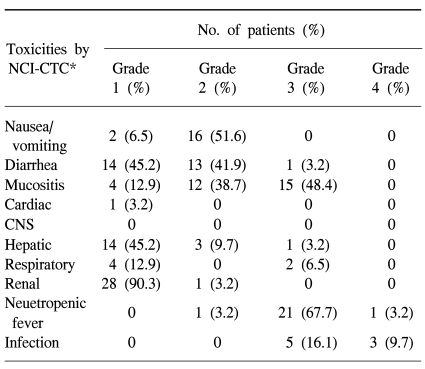
Early (within the first 100 days) treatment-related mortality occurred in three (9.7%) patients. Three patients died on days 6, 43 and 56, respectively, as a result of multiorgan failure associated with clinical sepsis syndrome involving E. coli (n=2) and an unknown organism (n=1). All early mortality patients were from the group with more advanced disease; two of the patients were previously heavily treated, had a primary refractory status, and one patient had required >3 regimens to achieve remission. The most common non-hematological toxicity among the total 31 patients was mucositis, with 15 patients (48.4%) developing grade 3 mucositis. Twenty-two (70.9%) patients developed neutropenic fever, and 8 patients had documented infections, with three of the latter developing sepsis (E. coli), one pneumonia, one acalculous cholecystitis with cholangitis (unknown organism) and three viral infections (herpes zoster). IPS >grade 3 developed in 2 patients; one died from progressed disease and pneumonia underlying IPS on day 110, and the other patient died at 25 months post transplantation. The latter patient had a history of systemic sclerosis prior to the NHL diagnosis, and such a combined autoimmune abnormality can aggravate IPS (19). There was no grade 3 or 4 CNS, hepatic, renal or cardiac toxicities (Table 2). No secondary leukemia or myelodysplastic syndrome developed.
Of the 5 patients that received involved filed radiation, one died due to IPS combined pneumonia and progressed disease at day 110.
Response to transplantation was evaluated 3 months after the procedure. Three patients were not evaluated due to an early death. Objective responses were obtained for 24 of 31 patients (77.4%), with complete remission (CR) in 19 patients (61.3%) and partial remission in 5 (16.1%) patients.
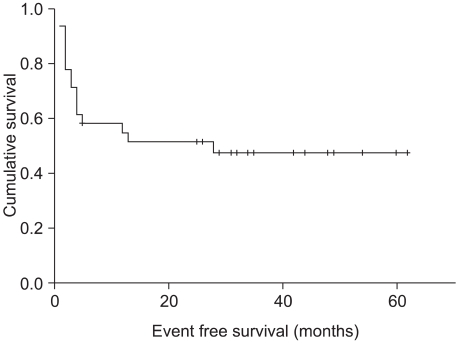
The median follow-up period in patients that underwent HDT and ASCT was 28 months (range 1~62 months). The 3-year cumulative estimate of OS and EFS rates were 62.3% and 47.3%, respectively. The OS did not reach the median, and the median EFS was 28 months (Fig 1, 2).
At the last follow up, 6 of the 19 patients in CR after transplantation had relapsed at a median of 4 months (range: 2~28 months) following transplantation. Of these 6 patients, 4 patients died and 2 were alive after relapses at 13 and 36 months. All of the patients received salvage chemotherapy. One of the relapsed patients received an allogenic PBSC transplantation, and has remained in the CR state. One patient underwent disease transformation into an acute lymphoblastic leukemia.
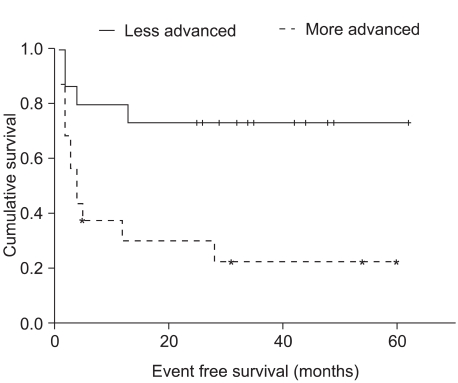
In the less advanced disease group (n=15), the 3-year OS and EFS rates were 73.3% and 73.3%, respectively, and did not reach the median. In the more advanced disease group (n=16), the 3-year OS and EFS rates were 51.9% and 22.5%, respectively. The median OS did not reach the median and the EFS was 4 months (95% CI 2.1~5.9 months). The EFS rate showed a significant difference in survival according to log rank testing (EFS rate: p=0.006) (Fig. 3).
In the less advanced disease group, response to HDT was CR in 11 (73.3%) patients. Two of these 11 patients relapsed at 4 months and 13 months. In the more advanced disease group, the response to HDT was CR in 6 patients (37.5%), PR in 3 patients (6.25%), and only two patients maintained CR at the last follow up. All three early treatment-related mortalities occurred in this group.
The superiority of HDT with ASCT compared with standard salvage chemotherapy in patients with refractory or relapsed NHL has been demonstrated in several randomized studies (2~3). However, the superiority of a specific conditioning regimen for NHL has not been demonstrated and only a few comparisons between regimens have been reported (5,6). TBI provides the following advantages: no cross resistance with chemotherapeutic agents, a homogenous dose of radiation administered to the body, and no sanctuary site sparing (20). Nevertheless, chemotherapy-only conditioning regimens are preferred due to concerns of increased acute toxicity and the possible risk of MDS/AML toxicity associated with TBI-based regimens (21).
Several studies comparing TBI-based conditioning regimens with chemotherapy alone showed no significant differences between the use of the two modalities (5,6). The 3- and 5-year EFS rates vary from 20~50% depending on the patient population evaluated (5~9). A study by Gutierrez-Delgado et al. compared patients receiving TBI/cyclophosphamide/etoposide with those receiving busulfan/melphalan and thiotepa, and had a median 5-year follow-up (6). The study found that the cumulative probabilities of survival, EFS and relapse at 5 years were 44%, 32% and 49%, with the TBI-containing regimen and 42%, 34% and 42%, respectively, with the chemotherapy-alone regimen (6). In the present study, the median follow-up duration was 28 months, and a longer follow-up period is warranted. The 3-year OS rate was 62.3%, and the 3-year EFS rate was 47.3% for all 31 patients. This result is consistent with previous reports.
In relapsed or refractory NHL patients, early treatment-related mortality (TRM) for TBI-based regimens has been reported to range from 4~34% (5~8, 22). TRM has decreased in recent years due to improvements in supportive care and the use of PBSC, although the TRM rate has been shown to be <10% in a recent report (8). Gutierrez-Delgado et al. reported a 13% TRM rate for the TBI-containing group of patients and 21% for the chemotherapy-alone group of patients. This relatively high TRM can be explained by the relatively small proportion of sensitive patients (only 42% of the entire group) (6). Stokerl-Goldstein et al. reported a 9% TRM rate, possibly reflecting that 125 (90%) of the 134 patients had sensitive disease (22). In the present study, the patients that experienced early TRM (9.7%; n=3) were previously heavily treated and had refractory disease. The inclusion of a significant number of patients (n=16, 51.6%) with more advanced disease could have affected the early TRM rate. High morbidity and toxicity were reported in patients that received prior dose-limiting radiation therapy and had more advanced disease (23). In this study, only 5 patients received dose-limiting radiation therapy, and one with primary refractory disease died by day 110. In the present study, the number of patients that received prior dose-limiting radiation therapy was small, and we are unable to draw conclusions regarding the effect of the dose-limiting radiation on morbidity and toxicity. We found that 6.5% (n=2) of all patients developed clinically significant interstitial pneumonitis syndrome. No VOD or later cardiac toxicity was observed. Thus, the TAM regimen appears to be relatively well tolerated.
Disease status at transplantation has consistently been identified as a prognostic factor for survival (5~8). In the present study, disease status at transplantation was the only factor affecting EFS. In terms of OS, a longer follow-up is warranted. Patients with less advanced disease had a higher 3-year EFS rate than patients with more advanced disease (p=0.006), and only one patient died from a cause other than relapse (6.7%). However, for less advanced disease, 9 patients with high-risk relapse disease were in the first CR. Among these patients, 3 patients had a DLBCL subtype, 3 patients had a PTCL subtype, 2 patients had Burkitt's lymphoma and 1 patient had a NK/T cell subtype. The use of front line HDT and auto transplantation as a consolidation approach for patients with a first CR of high risk NHL remains controversial, especially for patients with a DLBCL subtype. There is still some debate regarding the enrollment of these patients in HDT. However, less than 50% of the high risk patients are cured with standard therapy and consequently, these patients are appropriate candidates for experimental therapy. The final analysis of the GELA group LNH 87-2 trial showed a survival benefit for HDT in high risk patients as a consolidation approach (4). Therefore, a further prospective randomized trial is needed to confirm the benefit. In the patients with more advanced disease, the 3-year EFS of 22.5% was comparable to that seen in other series, but more than 70% of patients relapsed and all TRM occurred in this group. The present study found that the major cause of treatment failure in patients with more advanced disease was relapse. These findings indicate that the use of novel therapeutics should be explored in attempts to improve the outcome in such patients. Recently, the use of immunotherapy or radioimmunotherapy (Rituximab, 131I-tositumomab, 90Y-ibritumomab) as part of the conditioning regimen for ASCT has produced promising results with improved disease control (24, 25). The results of these newer approaches are expected to improve the outcome in these patients.
We report here that a modified TAM (fractionated TBI, high dose ARA-C and melphalan) conditioning regimen is a feasible HDT treatment following ASCT for clinically aggressive NHL. In less advanced disease patients, the 3-year EFS was 73.3%, and the regimen-related mortality was minimal. However, in more advanced disease patients, the 3-year EFS was only 22.5% and there was significant treatment-related mortality, indicating that this group of patient requires longer follow-up and will require the implementation of novel treatment strategies.
References
1. Armitage JO. Treatment of non-Hodgkin's lymphoma. N Engl J Med. 1993; 328:1023–1030. PMID: 8450856.

2. Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995; 333:1540–1545. PMID: 7477169.

3. Vose JM, Zhang MJ, Rowlings PA, Lazarus HM, Bolwell BJ, Freytes CO, et al. Autologous transplantation for diffuse aggressive non-Hodgkin's lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 2001; 19:406–413. PMID: 11208832.

4. Haioun C, Lepage E, Gisselbrecht C, Salles G, Coiffier B, Brice P, et al. Survival benefit of high-dose therapy in poor-risk aggressive non-Hodgkin's lymphoma: final analysis of the prospective LNH87-2 protocol--a groupe d'Etude des lymph omes de l'Adulte study. J Clin Oncol. 2000; 18:3025–3030. PMID: 10944137.
5. Salar A, Sierra J, Gandarillas M, Caballero MD, Marin J, Lahuerta JJ, et al. Autologous stem cell transplantation for clinically aggressive non-Hodgkin's lymphoma: the role of preparative regimens. Bone Marrow Transplant. 2001; 27:405–412. PMID: 11313670.

6. Gutierrez-Delgado F, Maloney DG, Press OW, Golden J, Holmberg LA, Maziarz RT, et al. Autologous stem cell transplantation for non-Hodgkin's lymphoma: comparison of radiation-based and chemotherapy-only preparative regimens. Bone Marrow Transplant. 2001; 28:455–461. PMID: 11593318.

7. Appelbaum FR, Sullivan KM, Buckner CD, Clift RA, Deeg HJ, Fefer A, et al. Treatment of malignant lymphoma in 100 patients with chemotherapy, total body irradiation, and marrow transplantation. J Clin Oncol. 1987; 5:1340–1347. PMID: 3305793.

8. Stein RS, Greer JP, Goodman S, Brandt SJ, Morgan DS, Macon WR, et al. Intensified preparative regimens and autologous transplantation in refractory or relapsed intermediate grade non-Hodgkin's lymphoma. t. Bone Marrow Transplant. 2000; 25:257–262. PMID: 10673696.
9. Weaver CH, Petersen FB, Appelbaum FR, Bensinger WI, Press O, Martin P, et al. High-dose fractionated total-body irradiation, etoposide, and cyclophosphamide followed by autologous stem-cell support in patients with malignant lymphoma. J Clin Oncol. 1994; 12:2559–2566. PMID: 7989929.

10. Bordigoni P, Esperou H, Souillet G, Pico J, Michel G, Lacour B, et al. Total body irradiation-high-dose cytosine arabinoside and melphalan followed by allogeneic bone marrow transplantation from HLA-identical siblings in the treatment of children with acute lymphoblastic leukaemia after relapse while receiving chemotherapy: a Societe Francaise de Greffe de Moelle study. Br J Haematol. 1998; 102:656–665. PMID: 9722290.
11. Dai QY, Souillet G, Bertrand Y, Galambrun C, Bleyzac N, Manel AM, et al. Antileukemic and long-term effects of two regimens with or without TBI in allogeneic bone marrow transplantation for childhood acute lymphoblastic leukemia. Bone Marrow Transplant. 2004; 34:667–673. PMID: 15354203.

12. Chopra R, McMillan AK, Linch DC, Yuklea S, Taghipour G, Pearce R, et al. The place of high-dose BEAM therapy and autologous bone marrow transplantation in poor-risk Hodgkin's disease. A single-center eight-year study of 155 patients. Blood. 1993; 81:1137–1145. PMID: 8443375.
13. Caballero MD, Rubio V, Rifon J, Heras I, Garcia-Sanz R, Vazquez L, et al. BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant. 1997; 20:451–458. PMID: 9313877.

14. Kim HJ, Min WS, Eom KS, Park SJ, Park YH, Kim DW, et al. Autologous stem cell transplantation using modified TAM or combination of triple-alkylating agents conditioning regi mens as one of the post-remission treatments in patients with adult acute myeloid leukemia in first complete remission. Bone Marrow Transplant. 2004; 34:215–220. PMID: 15170169.
15. Jaffe ES. World Health Organization. Pathology and genetics of tumours of haematopoietic and lymphoid tissue. 2001. Lyon: Oxford.
16. A predictive model for aggressive non-Hodgkin's lymphoma.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993; 329:987–994. PMID: 8141877.
17. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. NCI Sponsored International Working Group. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999; 17:1244. PMID: 10561185.

18. Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988; 6:1562–1568. PMID: 3049951.

19. Trad S, Amoura Z, Haroche J, Huong Du Boutin LT, Wechsler B, Leblond V, et al. Fatal progressive systemic sclerosis following autologous stem cell transplantation and high-dose chemotherapy. Rheumatology (Oxford). 2005; 44:951–953. PMID: 15814574.

20. Aristei C, Tabilio A. Total-body irradiation in the conditioning regimens for autologous stem cell transplantation in lymphoproliferative diseases. Oncologist. 1999; 4:386–397. PMID: 10551555.

21. Kalaycio M, Rybicki L, Pohlman B, Sobecks R, Andresen S, Kuczkowski E, et al. Risk factors before autologous stem-cell transplantation for lymphoma predict for secondary myelodysplasia and acute myelogenous leukemia. J Clin Oncol. 2006; 24:3604–3610. PMID: 16877727.

22. Stockerl-Goldstein KE, Horning SJ, Negrin RS, Chao NJ, Hu WW, Long GD, et al. Influence of preparatory regimen and source of hematopoietic cells on outcome of autotransplantation for non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 1996; 2:76–85. PMID: 9118302.
23. Crawford SW. Thomas ED, Forman SJ, Blume KG, editors. Critical care and resporiatory failure. Stem cell transplantation. Boston: Blackwell Scientific;p. 712–722.
24. Press OW, Eary JF, Gooley T, Gopal AK, Liu S, Rajendran JG, et al. Phase I /II trial of iodine-131-tositumomab (anti-CD20), etoposide, cyclophosphamide, and autologous stem cell transplantation for relapsed B-cell lymphomas. Blood. 2000; 96:2934–2942. PMID: 11049969.
25. Nademanee A, Forman S, Molina A, Fung H, Smith D, Dagis A, et al. A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgikin's lymphoma. Blood. 2005; 106:2896–2902. PMID: 16002426.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download