Abstract
Purpose
Recent studies have shown that Dickkopf-1 (DKK-1) is overexpressed in some tumors, including hepatocellular carcinoma. However, the role of increased DKK-1 in these tumors is not known. In this study, the DKK-1 expression in hepatocellular carcinoma (HCC) cell lines was evaluated and the effect of DKK-1 overexpression in HCC cell lines was studied.
Materials and Methods
The expression of DKK-1 in hepatocellular carcinoma cell lines was evaluated by RT-PCR. Stable cell lines that overexpressed DKK-1 were established. Cell growth, adhesion, migration and invasion assays were performed.
Results
RT-PCR analysis showed that 5 out of 8 HCC cell lines expressed DKK-1. The forced expression of DKK-1 suppressed the growth of cells and increased the population of cells in the sub-G1 phase. In addition, DKK-1 reduced the cellular adhesion capacity to collagen type I and fibronectin, and it increased migratory capacity. However, overexpression of DKK-1 did not increase the invasion capacity of the HCC cell line.
Wnt glycoproteins regulate cellular homeostasis and development by binding to membrane Frizzled-LRP5/6 receptor complexes (1). Wnt signaling includes a canonical pathway and noncanonical pathways. Canonical signaling causes accumulation of cytosolic β-catenin followed by translocation to the nucleus, where β-catenin acts as a co-activator of T-cell factor (TCF) proteins to regulate gene expression (1,2). Noncanonical pathways lead to the activation of Rho, Rac, JNK and PKC, or to changes in the levels of Ca2+ (1,2).
Dickkopf-1 (DKK-1) is a member of the DKK protein family that includes DKK-1, DKK-2, DKK-3 and DKK-4 (3). DKK-1 encodes a secreted Wnt antagonist that binds to LRP5/6 and so induces its endocytosis, leading to the inhibition of the canonical pathway (1). DKK-1 itself is a target of the beta-catenin/TCF signaling pathway (2,4).
The DKK-1 expression is downregulated in human colon cancer and it is transcriptionally silenced by CpG island promoter hypermethylation in colon cancer cell lines; this suggests that DKK-1 may act as a tumor suppressor (2,5). Consistent with this idea, DKK-1 suppresses cell growth and induces apoptotic cell death by activating the JNK pathway in human mesothelioma cells (6), and DKK-1 suppresses tumorigenicity in cervical carcinoma HeLa cells (7). In contrast, recent studies have shown that DKK-1 is overexpressed in hepatoblastoma, Wilms' tumors and hepatocellular carcinoma (8,9). However, the role of increased DKK-1 in these tumors is not known. Here, we examined the DKK-1 expression in hepatocellular carcinoma (HCC) cell lines and we studied the effect of DKK-1 overexpression in HCC cell lines.
Cell lines derived from hepatocellular carcinomas (HCCs) were purchased from the Korean Cell Line Bank (Seoul). Hep G2 and Hep 3B cells were grown in DMEM with 10% fetal bovine serum (FBS). The SNU 354, SNU 368, SNU 398, SNU 423, SNU 449 and SNU 475 cells (10) were cultured in RPMI 1640 with 10% FBS.
DKK-1 cDNA was prepared by RT-PCR and then it was subcloned into the EcoRI and XhoI sites of the pcDNA3.1/Myc-His (+) B vector (Invitrogen, Carlsbad, CA) in the sense orientation, generating pDKK-1/Myc-His. The orientation and accuracy of the sequences were confirmed by sequencing. The primers for PCR were 5'-GGAATTCCCTGAGATGATGGCTCTGGGCG-3' and 5' -CCGCTCGAGCGGTGTCTCTGACAAGTGTG-3'. The EcoRI (GAATTC) and XhoI (CTCGAG) sites are underlined.
The SNU 475 cells were grown to 50~80% confluence and then they were transfected with the control vector (pcDNA3.1/Myc-His (+) B vector) or with the pDKK-1/Myc-His vector with using Lipofectamine. After 2 days, the cell cultures were diluted 1 : 10 in selection medium (RPMI 10% medium with 400 µg/ml G418), and after 4 weeks, the individual and pooled clones were isolated and expanded.
cDNA was synthesized from 2 µg of the total RNA and small aliquots of the cDNA product were amplified with using the primers mentioned above. PCR amplification was performed after an initial denaturation for 5 min at 94℃, followed by 35 cycles for 30 s at 94℃, 45 s at 58℃ and 1 min at 72℃, and final elongation step was done at 72℃ for 10 min.
The total cell lysates were separated by 10% SDS-PAGE and then transferred to nitrocellulose membranes. The membranes were blocked with 6% milk in PBS for 1 h, and they were probed with mouse anti-β1-integrin antibody (BD Biosciences, Palo Alto, CA) and rabbit anti-β-catenin antibody (Cell Signaling, Beverly, MA). Horseradish peroxidase-conjugated sheep anti-mouse Ig (Amersham, UK) and donkey anti-rabbit Ig were used as the secondary antibodies at a 1 : 5,000 dilution, respectively. The bands were visualized using ECL Plus (Amersham). For the detection of DKK-1/Myc-His fusion protein in the culture supernatant, the conditioned media were collected and concentrated with using Centricon filters (10 kDa cut-off, Millipore, Bedford, MA); they were probed with mouse anti-c-myc-antibody (Roche, Germany).
The cells were plated at a density of 200,000 cells in 100 mm plates and they were incubated for 4 days. The viable cells were manually counted using a hemocytometer.
The cells were cultured in serum-free medium for 4 days. They were collected and washed with PBS and then fixed in 70% ethanol at 4℃ overnight. The cell cycle distribution was analyzed by flow cytometry as was previously described (11).
The wells of 96-well plates were coated with 10 µg/ml Type I collagen or fibronectin overnight at 4℃, and they were blocked with 1.5% BSA for 1.5 h at 37℃. The cells were plated at a density of 20,000 cells/well and then incubated in serum-free medium for 24 h. They were then washed twice in PBS, fixed with 4% paraformaldehyde for 10 min, and stained with 0.1% crystal violet for 20 min. Thereafter, the stained cells were washed with PBS, air-dried and solubilized in 50 µl of 100 mM HCl; the optical density was measured at 630 nm.
30,000 cells in 100 µl of RPMI medium were added to the upper compartment of MiliCell chambers that had 8 µm pores, and the chambers were placed into 24-well culture dishes that contained 500 µl of 10% RPMI medium. After 2 h at 37℃, the cells on the upper side of the filter were removed with a cotton swab and those attached to the underside were stained with 0.1% crystal violet; following this, they were photographed and counted. For the invasion assays, growth factor-reduced Matrigel (BD Biosciences) was diluted (30 µg in 100 µl of H2O); this was added to the top chamber and allowed to gel for 1 h at 37℃, air-dried for 16 h, and the Matrigel barrier was reconstituted for 2 h at 37℃ prior to use. After 24 h at 37℃, cells were counted in the same manner as for the migration assay.
We investigated the DKK-1 expression in 8 human hepatocellular carcinoma cell lines by performing RT-PCR analysis. As shown in Fig. 1, we identified a DKK-1 expression in 63% (5/8) of the HCC cell lines. The Hep G2, Hep 3B, SNU 398 and SNU 449 cells expressed high levels of DKK-1. The SNU 368 cells expressed a moderate level of DKK-1, whereas the SNU 354, SNU 423 and SNU 475 cells expressed very low or no detectable levels of DKK-1.
To assess the role of the overexpressed DKK-1 in the biology of hepatocellular carcinoma cells, we transfected SNU 475 cells with a DKK-1 expression vector (pDKK-1/Myc-His). The individual G418 resistant clones were isolated after a few weeks and the expression of DKK-1 was examined. We eventually selected two clones (Clone #19 and Clone #22) for further study. SNU 475 cells were also transfected with an empty vector (pcDNA3.1/Myc-His (+) B) and the G418 resistant clones were pooled and named as the 'control'. The Clone #19 cells expressed a higher level of DKK-1 mRNA than did the Clone #22 cells (Fig. 2A). Consistent with this, Clone #19 secreted higher levels of DKK-1/Myc-His fusion protein into the culture medium (Fig. 2B). A smeared signal around 35 KDa on the immunoblotting indicates that the secreted DKK-1 fusion protein is subjected to post-translational modification, as is the case for the wild-type protein (3,12). The SNU 475 cells expressing DKK-1 displayed a significantly reduced level of β-catenin, demonstrating the inhibition of the canonical Wnt pathway by DKK-1 fusion protein, and this resulted in β-catenin destabilization (Fig. 2C).
Measurements of the effect of DKK-1 on cell growth revealed that cell growth was suppressed in the clones that expressed DKK-1 (Fig. 3A). The Clone #19 cells expressing a higher level of DKK-1 showed more suppression than did the Clone #22 cells. In addition, the clones expressing DKK-1 were more susceptible to apoptosis, as evidenced by the increased population of cells in the sub-G1 phase, which represented apoptotic cells (Fig. 3B).
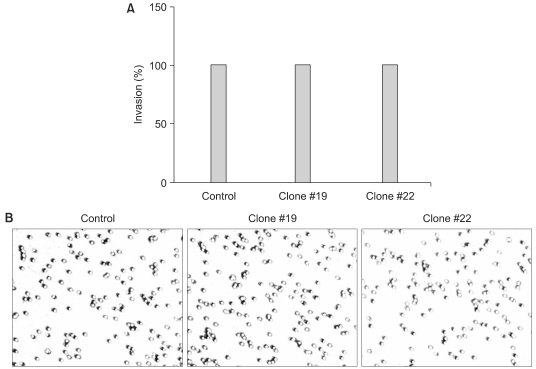
We examined whether the forced expression of DKK-1 alters cell-to-matrix adhesion. The SNU 475 cells expressing DKK-1 displayed significantly reduced adhesion to both collagen type I and fibronectin (Fig. 4A, B). In line with this finding, we found that β1-integrin, which is a transmembrane receptor that binds to fibronectin and collagen, is significantly decreased in the cells expressing DKK-1 (Fig. 4C). We also checked whether DKK-1 affects cell motility and invasiveness. The DKK-1 expressing cells (Clone #19 and #22) had a greater migratory capacity than did the control cells (Fig. 5). However, cellular invasiveness was not affected by DKK-1, as examined by the invasion assay (Fig. 6).
In this study, we observed that DKK-1 is expressed in 63% (5/8) of the HCC cell lines and the overexpression of DKK-1 caused reduced cell adhesion as well as increased cell migration, which are the hallmarks of tumor promotion. Our findings are in conflict with recent reports (2,5) in which the DKK-1 expression is downregulated in human colon cancer and it is transcriptionally silenced by CpG island promoter hypermethylation in colon cancer cell lines, which suggests that DKK-1 may act as a tumor suppressor. However, our finding is in line with the overexpression of DKK-1 in hepatoblastoma, Wilms' tumors and hepatocellular carcinoma (8,9).
Because DKK-1 is one of the target genes of the canonical Wnt/β-catenin signaling pathway (4), the overexpression of DKK-1 in some HCC cell lines may represent a negative feedback mechanism of uncontrolled Wnt signaling, as has been suggested in the case of hepatoblastoma and Wilms' tumors (8). In this regard, it is worthy to note that activatingβ-catenin mutations have been found in 8 of 31 HCCs (13), and some subgroups of HCCs are strongly related to β-catenin mutations (14). Also, it has been reported that aberrant nuclear accumulation of β-catenin is observed in ~30~40% of HCCs (15~17) and the Wnt receptor frizzled-7 is overexpressed in human HCC (18), leading to activation of the Wnt pathway.
We also observed that the forced expression of DKK-1 suppresses the growth of SNU 475 cells, and the cells expressing DKK-1 were more susceptible to apoptosis, as evidenced by the increased population of cells in the sub-G1 phase. These results are in line with the finding that DKK-1 overexpression in brain tumor cells and HeLa cervical carcinoma cells increases their sensitivity to apoptosis following alkylation damage of their DNA and UV irradiation, respectively (7,19).
The decreased adhesion to fibronectin and collagen by DKK-1 expressing cells is thought to be due, at least in part, to the down-regulation of β1-integrin. This is in line with the finding that HeLa cells expressing DKK-1 had a ~30% reduction in plating efficiency (7). It is also intriguing that DKK-1 increases cellular migration. We guess it is possible that the increased migration of cells expressing DKK-1 may be due to the reduced level of β-catenin by inhibition of the canonical Wnt pathway via DKK-1 (Fig. 2C). In this regard, it is interesting to note that β-catenin not only plays a role as a coactivator of TCF proteins, but it also binds directly to the intracellular domain of E-cadherin that mediates adhesion between cells (20). On the other hand, DKK-1 did not increase cellular invasiveness. This result suggests that DKK-1 does not affect synthesis of proteases such as matrix metalloprotease-2 (MMP-2) that acts on collagen type IV, a major component of the Matrigel matrix used in this study. We have also found, by performing RT-PCR analysis, that the level of MMP-2 did not change in Clones #19 and #22 (data not shown).
In this study, we found that DKK-1 is expressed in some HCC cell lines and the forced expression of DKK-1 caused growth inhibition. On the other hand, the overexpression of DKK-1 resulted in reduced cell adhesion as well as increased cell migration, which are the hallmarks of tumor promotion. Collectively, our data suggest that overexpression of DKK-1 affects the biology of HCC cells, although further in vivo studies are needed to confirm the in vitro data presented here.
References
1. Miler JR. The Wnts. Genome Biol. 2002; 3:REVIEWS3001. PMID: 11806834.
2. Gonzalez-Sancho JM, Aguilera O, Garcia JM, Pendas-Franco N, Pena C, Cal S, et al. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005; 24:1098–1103. PMID: 15592505.
3. Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999; 238:301–313. PMID: 10570958.

4. Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, et al. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004; 23:8520–8526. PMID: 15378020.
5. Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, Espada J, et al. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006; 25:4116–4121. PMID: 16491118.

6. Lee AY, He B, You L, Xu Z, Mazieres J, Reguart N, et al. Dickkopf-1 antagonizes Wnt signaling independent of beta-catenin in human mesothelioma. Biochem Biophys Res Commun. 2004; 323:1246–1250. PMID: 15451431.
7. Mikheev AM, Mikheeva SA, Liu B, Cohen P, Zarbl H. A functional genomics approach for the identification of putative tumor suppressor genes: Dickkopf-1 as suppressor of HeLa cell transformation. Carcinogenesis. 2004; 25:47–59. PMID: 14555616.

8. Wirths O, Waha A, Weggen S, Schirmacher P, Kuhne T, Goodyer CG, et al. Overexpression of human Dickkopf-1, an antagonist of wingless/WNT signaling, in human hepatoblastomas and Wilms' tumors. Lab Invest. 2003; 83:429–434. PMID: 12649343.

9. Patil MA, Chua M, Pan K, Lin R, Lih C, Cheung S, et al. An integrated data analysis approach to characterize genes highly expressed in hepatocellular carcinoma. Oncogene. 2005; 24:3737–3742. PMID: 15735714.

10. Park JG, Lee JH, Kang MS, Park KJ, Jeon YM, Lee HJ, et al. Characterization of cell lines established from human hepatocellular carcinoma. Int J Cancer. 1995; 62:276–282. PMID: 7543080.

11. Farooq M, Hwang SY, Park MK, Kim JC, Kim MK, Sung YK. Blocking endogenous glypican-3 expression releases Hep 3B cells from G1 arrest. Mol Cells. 2003; 15:356–360. PMID: 12872992.
12. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998; 391:357–362. PMID: 9450748.

13. de la Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carci nomas. Proc Natl Acad Sci USA. 1998; 95:8847–8851. PMID: 9671767.
14. Boyault S, Rickman DS, de Reynies A, Balabaud C, Rebouissou S, Jeannot E, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007; 45:42–52. PMID: 17187432.

15. Kondo Y, Kanai Y, Sakamoto M, Genda T, Mizokami M, Ueda R, et al. Beta-catenin accumulation and mutation of exon 3 of the beta-catenin gene in hepatocellular carcinoma. Jpn J Cancer Res. 1999; 90:1301–1309. PMID: 10665646.
16. Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM, et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. 2002; 21:4863–4871. PMID: 12101426.
17. Park JY, Park WS, Nam SW, Kim SY, Lee SH, Yoo NJ, et al. Mutations of beta-catenin and AXIN I genes are a late event in human hepatocellular carcinogenesis. Liver Int. 2005; 25:70–76. PMID: 15698401.
18. Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, et al. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004; 127:1110–1122. PMID: 15480989.

19. Shou J, Ali-Osman F, Multani AS, Pathak S, Fedi P, Srivenugopal KS. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene. 2002; 21:878–889. PMID: 11840333.

20. Aplin AE, Howe AK, Juliano RL. Cell adhesion molecules, signal transduction and cell growth. Curr Opin Cell Biol. 1999; 11:737–744. PMID: 10600702.

Fig. 1
DKK-1 expression in the HCC cell lines. (A) RT-PCR analysis of the DKK-1 transcript. (B) The relative DKK-1 expression levels as measured from the RT-PCR analysis by densitometry.
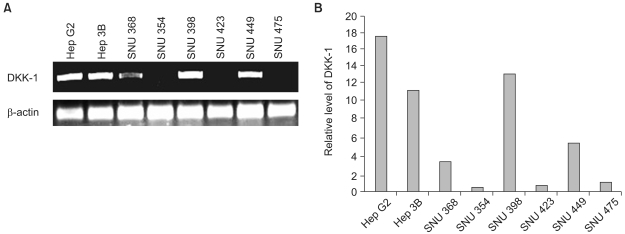
Fig. 2
Forced expression of DKK-1 reduces the β-catenin level in SNU 475 cells. (A) RT-PCR analysis of the DKK-1 transcript. (B) Immunoblot analysis of the DKK-1/Myc-His fusion protein in the culture medium. (C) Immunoblot analysis of the β-catenin expression. Control: SNU 475 cells transfected with empty vector, Clones #19 and #22: clones transfected with vector pDKK-1/Myc-His.
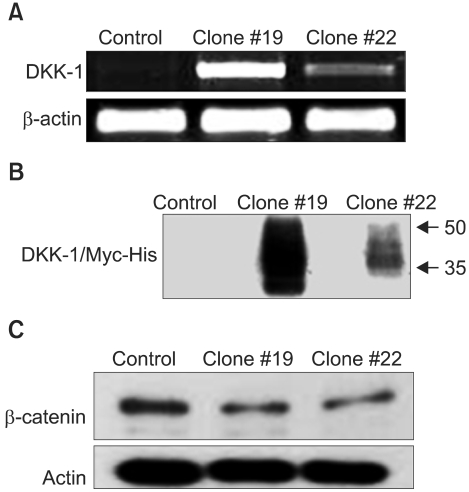
Fig. 3
Growth suppression by DKK-1. (A) Direct counting. Values represent the means±standard error of 2 determinations per experiment from 3 independent experiments over 4 days of culture (*p<0.05 compared to the control). (B) A representative DNA histogram. Cells were stained with propidium iodide and analyzed by flow cytometry. The percentage of cells in the sub-G1 phase is shown.

Fig. 4
DKK-1 reduces cell-to-matrix adhesion. The cells were seeded on wells coated with collagen type I or fibronectin. The attached cells were stained with crystal violet and the optical density was measured at 630 nm (A) and the cells were photographed under a microscope (B). Values represent the means±standard error of 5 determinations per experiment from 3 independent experiments (*p<0.05 compared to the control). (C) Immunoblot analysis of β1-integrin, a transmembrane receptor that binds to fibronectin and collagen. Probing with anti-actin antibody confirmed equal loading and transfer.
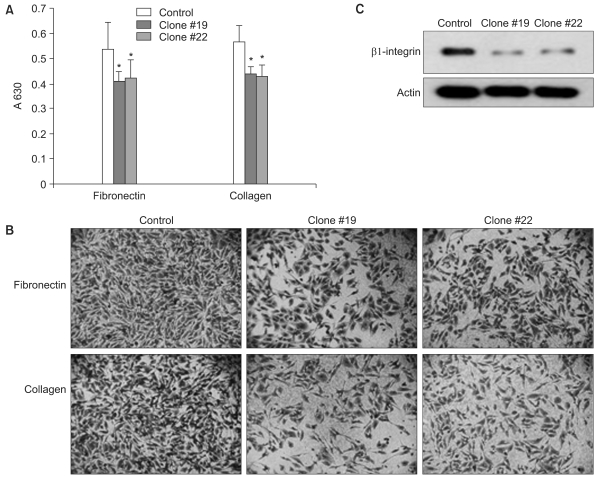
Fig. 5
DKK-1 increases the cells' migratory capacity. The cells were grown on transwells and the cells that had migrated to the underside of the transwells were stained and counted (A), and then photographed under a microscope (B). The values represent the means±standard error of 3 determinations.
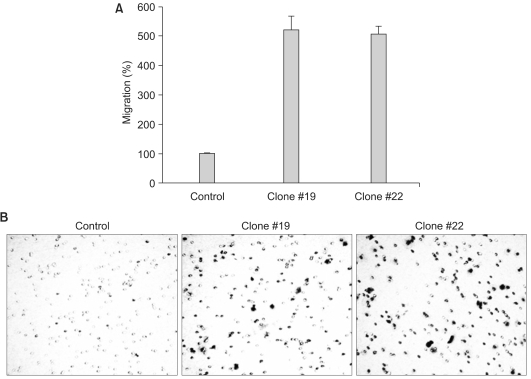
Fig. 6
DKK-1 does not increase the cells' invasion capacity. The cells were grown on Matrigel-coated transwells and the cells that had invaded to the underside of the transwells were stained and counted (A), and then photographed under a microscope (B). The values represent the means±standard error of 2 determinations.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download