Abstract
One of the most significant developments in medical oncology practice has been the approval of various antiangiogenic drugs for the treatment of a number of different malignancies. These drugs include bevacizumab (Avastin®), the anti-VEGF monoclonal antibody. Thus far, bevacizumab appears to induce clinical benefit in patients who have advanced metastatic disease only or primarily when it is combined with conventional chemotherapy. The reasons for the chemo-enhancing effects of bevacizumab are unknown, and this is a subject that we have been actively studying along with additional ways that antiangiogenic drugs may be combined with chemotherapy. In this respect, we have focused much of our effort on metronomic low dose chemotherapy. We have been studying the hypothesis that some chemotherapy drugs at maximum tolerated doses or other cytotoxic- like drugs such as acute "vascular disrupting agents" (VDAs) can cause an acute mobilization of proangiogenic cells from the bone marrow which home to and colonize the treated tumors, thus accelerating their recovery. These cells include endothelial progenitor cells. This systemic process can be largely blocked by a targeted antiangiogenic drug, e.g. anti-VEGFR-2 antibodies. In addition, metronomic chemotherapy, i.e., close regular administration of chemotherapy drugs at low non-toxic doses with no breaks, over prolonged periods of time not only prevents the acute CEP bone marrow response, but can even target the cells. This potential antiangiogenic effect of metronomic chemotherapy can also be boosted by combination with a targeted antiangiogenic agent. Treatment combinations of metronomic chemotherapy and an antiangiogenic drug have moved into phase II clinical trial testing with particularly encouraging results thus far reported in metastatic breast and recurrent ovarian cancer. Oral chemotherapy drugs such as cyclophosphamide (CTX), methotrexate are the main chemotherapeutics used for such trials. Oral 5-FU prodrugs such as UFT would also appear to be highly suitable based on long term adjuvant therapy studies in patients. Recent preclinical results using metronomic cyclophosphamide and metronomic UFT in models of advanced metastatic breast cancer suggest that this type of combination might be particularly promising for metronomic chemotherapy in this indication, particularly when combined with a targeted antiangiogenic drug.
A recent major breakthrough in medical oncology has been the demonstration of efficacy in randomized phase III clinical trials of antiangiogenic drugs, in particular bevacizumab (Avastin®), the 'targeted' anti-VEGF monoclonal antibody, when combined with standard chemotherapy. Such trials have shown clinical benefit (in progression free survival or overall survival) in advanced colorectal cancer, advanced non small cell lung cancer, as well as for response rate and progression free survival in metastatic breast cancer (1). How a "tumor starving" drug such as bevacizumab enhances the efficacy of conventional chemotherapy remains something of a mystery although there are a number of different theories currently being studied (1,2). Also interesting is that chemotherapy itself may have effects on tumor angiogenesis (3,4) either by targeting dividing endothelial cells present in the tumor's growing neovasculature (a local antiangiogenic effect), or by mobilizing bone marrow derived proangiogenic cells such as circulating endothelial progenitor cells (CEPs) - a systemic and potentially proangiogenic effect (3). However, it appears that an overall significant antiangiogenic effect is obtained when chemotherapy is administered in a so-called "metronomic" fashion (3), the extent of which can be amplified by concurrent combination with an antiangiogenic drug such as VEGF or VEGF receptor targeting antibodies.
Metronomic chemotherapy refers to the close, regular administration of a chemotherapeutic drug at relatively low (non-toxic) doses, over prolonged periods, with no extended drug-free break periods (5). As such it can be viewed as a form of "dose dense" chemotherapy but differs from most forms of the latter in several ways (3). First, it is not necessarily "dose intense" where the goal remains to deliver greater cumulative levels of drug, at shortened intervals, e.g. once every two weeks instead of once every three weeks. Second, because metronomic regimens are much less toxic, they do not usually require hematopoietic growth factor support (e.g. using recombinant G-CSF), or other supportive care measures (6,7). Metronomic chemotherapy can thus be viewed as a form of long term 'maintenance' chemotherapy that can be used on its own (3,5), or perhaps more importantly, combined over long periods of time with biologic targeted therapies, especially antiangiogenic drugs such as anti-VEGFR-2 antibodies (8) or small molecule multi-targeted VEGFR-2 antagonist receptor tyrosine kinase inhibitors (9) and possibly other drugs or therapies such as trastuzumab (Herceptin®) (10), tumor vaccines (11), or even aromatase inhibitors such as letrozole (12). It can also be integrated and sequenced with standard MTD type chemotherapy where brief courses of such induction therapy, given 'upfront', is followed by long term maintenance/metronomic low-dose chemotherapy (sometimes called "chemo-switching") (9) combined with a concurrent targeted therapy (8,9). Among the advantages of metronomic chemotherapy include reduced acute toxicities such as high grade myelosuppression, vomiting, nausea, mucositis, etc. (6,7), sometimes surprisingly good anti-tumor activity (based mostly on preclinical studies so far), even when used to treat drug resistant tumors (5), reduced costs when using off patent chemotherapeutic drugs (13), and increased convenience when using oral drugs which can be taken at home (3,6). These potential advantages could be useful for long term adjuvant therapy of early stage cancers. Long term daily oral administration of a drug such as UFT (an oral 5-FU prodrug) for 2 years with no breaks might be viewed as an example, in retrospect, of metronomic chemotherapy (14). This current major disadvantage of translating metronomic chemotherapy into the clinic is the empiricism associated with determining the optimal biologic dose (OBD).
There is growing interest in the idea that chemotherapy drugs can inhibit tumor angiogenesis, and that this 'side effect' of chemotherapy may contribute to its anti-tumor activity, in addition to direct cytotoxic effects on tumor cells. The basis for the hypothesis that chemotherapy can target tumor angiogenesis originally stemmed from the observation that a hallmark of angiogenesis is the presence of dividing endothelial cells in sprouting blood vessel capillaries (15,16). Such cells should be sensitive to most chemotherapy drugs which are designed to preferentially target cycling cells (4,17). Indeed there is evidence that most known chemotherapy drugs can suppress angiogenesis using a variety of commonly used in vivo assays (4). Indeed if a chemotherapy drug such as cyclophosphamide is given in cycles of maximum tolerated dose (MTD) therapy separated by long drug -free intervals (to allow recovery from some of the toxic effects of such therapy such as myelosuppression) to tumor-bearing mice, there is evidence of rapidly-induced endothelial cell apoptosis in the tumor's vasculature. But this does not necessarily lead to the expected antiangiogenic effect. The reason is that this damage to the tumor endothelium can be largely and rapidly reversed during the extended drug free break periods (5), perhaps by a massive hemopoiesis-like mobilization ('rebound') of circulating endothelial progenitor cells (CEPs) from the bone marrow (18) (Fig. 1) which could conceivably then home to the damaged tumor endothelium and set about repairing the damage (CEPs are discussed in more detail in the next section). Thus shortening the break periods is critical to ensuring the repair process is prevented or minimized (5). This in turn requires relatively low doses of drug to be used for each drug treatment (5). These results may also help explain why, in some cases, an antiangiogenic drug such as bevacizumab increases the efficacy of standard maximum tolerated dose chemotherapy: the acute systemic CEP rebound occurring during the break periods, which is also observed clinically (19), would presumably be suppressed or blocked, thus preventing a potential 'reactive' pro-tumor angiogenic response (2). Another mechanism, put forward by Hudis (20) is that the local tumor cell repopulation/rebound that occurs after MTD chemotherapy would be presumably slowed by the presence of an antiangiogenic agent, since repopulating tumor cells would require optimal oxygen and nutrients supplied by the tumor vasculature for optimal (re)growth. Thus blocking angiogenesis would be expected to increase progression free and/or overall survival times in such circumstances. In summary, MTD chemotherapy can have an initial local (i.e., intra-tumoral) antiangiogenic effect but this is quickly followed by a reactive and potentially proangiogenic (CEP) systemic effect which may negate the initial antiangiogenic local effect. However, the latter proangiogenic effect can be blocked by use of an antiangiogenic drug such as bevacizumab along with MTD chemotherapy; overall maximal and sustained inhibition of tumor angiogenesis can be achieved by using metronomic chemotherapy regimens combined with a targeted antiangiogenic drug.
The aforementioned results highlight the possible impact that bone marrow-derived circulating progenitor cells might have a significant impact on response to chemotherapy and/or antiangiogenic drugs. As such the concept of CEPs deserve special mention.
Until 1997, it was generally accepted that all new endothelial cells in a sprouting (angiogenic) vessel capillary were derived from the division of pre-existing differentiated endothelial cells. However, Isner and colleagues that year reported results showing that up to 20% of the endothelial cells in such vessels were derived from circulating bone marrow cells (21). The methodology used to arrive at such a startling figure was the use of lethally irradiated mice transplanted with syngeneic bone marrow cells which expressed a marker that could distinguish between host and donor bone marrow cell populations, e.g. β-galactosidase (lacZ). A similar methodology has been employed using bone marrow cells transfected with the gene encoding green fluorescent protein (GFP). Such bone marrow chimeric mice are then used as recipients for a tumor transplant, and the tumor associated blood vessels of such tumors carefully examined for the presence of the donor bone marrow derived cell populations within the tumor vasculature using appropriate microscopy techniques which, if properly done, can be used to detect luminally incorporated endothelial cells (derived from the donor bone marrow population). Over the ensuing 10 years many studies have been published utilizing these and similar techniques; the results of such studies have been quite variable in terms of the percentages of bone marrow derived cells incorporated into the lumens of growing tumor-associated blood vessels (22,23). Most studies show much lower percentages than originally reported by Isner and colleagues (e.g. 1~5%) although there are some conspicuous exceptions (where the numbers can be even higher, e.g. 50%) (24,25). This has raised doubts about the impact and relevance of CEPs to tumor angiogenesis (22,23). However, all these studies involved analysis of untreated tumors. As discussed above, the situation might change suddenly and dramatically after therapeutic intervention using certain drugs such as MTD cyclophosphamide which have the ability to cause an acute and massive mobilization of bone marrow derived endothelial progenitor cells, as reported previously (18) and illustrated in Fig. 1.
We hypothesized that such acutely mobilized CEPs might subsequently home to drug treated tumors in large numbers and then facilitate tumor repopulation, primarily by amplifying tumor angiogenesis. Indeed, evidence consistent with this hypothesis was reported using a type of vascular targeting drug known as VDAs - "vascular disrupting agents" (26). These drugs, which are mostly microtubule inhibitors, can cause an acute drop in tumor blood flow subsequent to targeting and destroying the established tumor vasculature and thus causing massive intra-tumoral hypoxia and tumor cell necrosis (27). However, a viable rim of tumor tissue almost always remains following VDA therapy from which rapid tumor regrowth ensues. We recently reported a massive invasion and colonization of tumors by bone marrow derived CEPs is induced by VDA therapy (Fig. 2). These cells colonize the viable tumor rim remaining after VDA therapy and moreover contribute to regrowth of the tumors (26), a process that can be blocked by combination treatment using an antiangiogenic drug, e.g. a VEGFR-2 neutralizing monoclonal antibody (DC101), along with the VDA (Fig. 2). More recently, we have observed similar results using a variety of chemotherapy drugs, i.e., acute induction of CEPs which home to the chemotherapy drug treated tumors within a matter of hours to days after a single injection of certain chemotherapy drugs (Shaked et al., unpublished observation). These studies were undertaken after another series of experiments were completed that were designed to determine whether CEPs could be used as a surrogate biomarker for angiogenesis and hence antiangiogenic drug therapy. This was indeed found to be the case based on both pharmacologic and genetic experiments (26). Thus we were able to show that measurement of CEPs in the peripheral blood of mice could help determine the OBD range for targeted biologic antiangiogenic drugs such as DC101 (22).
With respect to clinical application, there is at least one significant disadvantage to metronomic chemotherapy as mentioned above: the empiricism associated with determining the optimal 'low' dose, i.e., the optimal biologic (therapeutic) dose (OBD). Because the predominant mechanism for metronomic chemotherapy is thought to be antiangiogenesis (3,5,8) (although other additional mechanisms may be involved as well, such stimulation of the host immune system (28), as a result of targeting both dividing endothelial cells in the tumor's growing vasculature (5) and CEPs (18), we investigated whether these cells could be used as a surrogate marker in mice for determining the OBD for various metronomic chemotherapy regimens (29). The results, an example of which is shown in Fig. 3, showed CEPs can indeed be used successfully for such a purpose in mice (22,29-31). Thus, we can reduce the level of empiricism (at least in mice) when testing metronomic chemotherapy regimens, with respect to determining the OBD.
Whether CEPs can be used as a similarly reliable pharmacodynamic surrogate marker in the clinical setting is problematic given their lower levels in humans compared to mice (23). However, evidence has been obtained recently showing that circulating endothelial cells (CECs) which are apoptotic may be a potentially useful biomarker for metronomic chemotherapy biologic activity (32). Preclinical studies suggest that the likely source of such cells is the tumor vasculature since increases in apoptotic CECs are not detected when normal mice are treated with metronomic chemotherapy (32). However, there is no evidence to show that such cells can be used to help establish the OBD for metronomic chemotherapy in mice or human.
An example of the benefits of reducing the empiricism of metronomic chemotherapy using CEPs to help establish the OBD can be seen from preclinical experiments determining the anti-tumor effects of single versus two chemotherapeutic drug combinations, administered in a metronomic fashion, i.e., daily (by oral delivery) in a model of advanced high volume (end stage) visceral metastatic disease of human breast cancer xenografts where therapy was initiated at terminal stages of disease (31). One drug was cyclophosphamide administered at 20 mg/kg/day - the OBD determined by effects on CEPs, administered through the drinking water (33). The other drug used was UFT administered by gavage every day for 140 days (with or without cyclophosphamide) at the determined OBD, namely, 15 mg/kg (31).
The rationale for undertaking these studies was as follows. First, it is often the case that doublet combinations of chemotherapy, when administering such drugs in an MTD fashion, is superior to mono-chemotherapy in the clinical setting. Second, it is rare for preclinical investigators to undertake studies involving therapy of advanced metastatic disease despite the fact that most phase I and phase II clinical trials involve treatment of such patients. It is well known that it is usually much more difficult to treat advanced metastatic disease, as compared to earlier stage microscopic metastatic disease or primary tumors (34,35). Consequently, this may be a major source for the discrepancy that is often observed between preclinical studies testing various therapies for anti-tumor activity in mice to what is subsequently observed when similar drugs/therapies are tested in cancer patients (34,35). The usual trend is that highly encouraging results in mice are not reproduced in the clinical setting. We reasoned that this discrepancy might be partially resolved by undertaking studies of advanced metastatic cancer in mice and moreover, there might be occasional exceptions to the rule that advanced metastatic disease is much less responsive, or resistant altogether, to a particular anti-cancer therapy. Discovery of such exceptions could be followed by their assessment in the clinical setting, with a great degree of confidence that significant anti-tumor activity might be observed.
With this aforementioned rationale in mind, it was encouraging that we observed truly remarkable long-term survival effects when the combination treatment of metronomic UFT plus metronomic cyclophosphamide was used, in comparison to either drug treatment alone (31) (Fig. 4, 5). The combination of a 5-FU prodrug, e.g. capecitabine and metronomic cyclophosphamide has now advanced to clinical trial testing in the setting of metastatic breast cancer where this combination is being used in combination with bevacizumab in an ongoing phase II clinical trial being undertaken in Milan, Italy at the European Institute of Oncology (36).
Also of considerable interest with respect to our UFT plus cyclophosphamide preclinical results was the observation that the combination treatment did not have a compelling anti-tumor effect when used to treat primary localized tumors using the same tumor cell lines, transplanted into the mammary fat pad. Indeed the UFT treatment alone did not seem to have any effect in this setting, did not enhance the anti-tumor effect of metronomic cyclophosphamide (Fig. 6). However, subsequent careful analysis revealed that the UFT treatment did have an effect on inhibiting local invasion of the primary tumors as well as inhibiting distant microscopic metastases (31). Thus two important implications emerge from these results. First, lack of response of primary tumors - as measured only by tumor volume changes - may mask other anti-tumor effects that are more subtle, yet biologically important. Second, there may be situations, surprisingly, where high volume metastases may respond better to a particular therapy compared to control primary tumors.
Because of these findings we have embarked on a major program developing models of advanced metastatic cancer in mice, using human tumor cell lines, that are then used for therapy studies.
Metronomic chemotherapy regimens have moved into phase II clinical trial testing both in the adjuvant and metastatic settings where they are often combined with a targeted antiangiogenic drug such as bevacizumab (Avastin®), or another targeted agent that likely has some antiangiogenic activity, e.g. the COX-2 inhibitor, celecoxib (Celebrex) (37) or other agents such as letrozole (12) (Table 1). Some interim results of phase II metronomic chemotherapy clinical trials using bevacizumab and daily low dose oral cyclophosphamide in metastatic breast cancer or advanced, recurrent ovarian cancer look extremely promising (38). Ongoing, planned or recently completed (6,39) phase II clinical trials of metronomic chemotherapy, especially those combined with an agent such as bevacizumab should indicate whether or not this treatment strategy has promise in the treatment of metastatic and/or early stage human cancer.
ACKNOWLEDGEMENTS
RSK wishes to thank Cassandra Cheng for her excellent secretarial assistance and to the numerous people in his lab and external collaborators involved in the work summarized in this review, and published in numerous reviewed publications as well as reviews.
References
1. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005; 438:967–974. PMID: 16355214.

2. Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006; 312:1171–1175. PMID: 16728631.

3. Kerbel RS, Kamen BA. Antiangiogenic basis of low-dose metronomic chemotherapy. Nature Rev Cancer. 2004; 4:423–436. PMID: 15170445.
4. Miller KD, Sweeney CJ, Sledge GW Jr. Redefining the target: chemotherapeutics as antiangiogenics. J Clin Oncol. 2001; 19:1195–1206. PMID: 11181686.

5. Browder T, Butterfield CE, Kraling BM, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000; 60:1878–1886. PMID: 10766175.
6. Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nole F, et al. Low dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002; 13:73–80. PMID: 11863115.
7. Emmenegger U, Man S, Shaked Y, Francia G, Wong JW, Hicklin DJ, et al. A comparative analysis of low dose metronomic cyclophosphamide reveals absent or low grade toxicity on tissues highly sensitive to the toxic effects of maximum tolerated dose regimens. Cancer Res. 2004; 64:3994–4000. PMID: 15173013.
8. Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin D, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000; 105:R15–R24. PMID: 10772661.

9. Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose "chemo-switch" regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005; 23:939–952. PMID: 15557593.

10. du Manoir JM, Francia G, Man S, Mossoba M, Medin JA, Viloria-Petit A, et al. Strategies for delaying or treating in vivo acquired resistance to trastuzumab (Herceptin®) in human breast cancer xenografts. Clin Cancer Res. 2006; 12:904–916. PMID: 16467105.
11. Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003; 63:8408–8413. PMID: 14679003.
12. Bottini A, Generali D, Brizzi MP, Fox SB, Bersiga A, Bonardi S, et al. Randomized phase II trial of letrozole and letrozole plus low-dose metronomic oral cyclophosphamide as primary systemic treatment in elderly breast cancer patients. J Clin Oncol. 2006; 24:3623–3628. PMID: 16877730.

13. Bocci G, Tuccori M, Emmenegger U, Liguori V, Kerbel RS, Del Tacca M. Cyclophosphamide-methotrexate "metronomic" chemotherapy for the palliative treatment of metastatic breast cancer. A comparative pharmacoeconomic evaluation. Ann Oncol. 2004; 16:1243–1252. PMID: 15905308.

14. Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, et al. A randomized trial of adjuvant chemotherapy with uraciltegafur for adenocarcinoma of the lung. N Engl J Med. 2004; 350:1713–1721. PMID: 15102997.

15. Tannock IF. Population kinetics of carcinoma cells, capillary endothelial cells, and fibroblasts in a transplanted mouse mammary tumor. Cancer Res. 1970; 30:2470–2476. PMID: 4097429.
16. Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000; 60:1388–1393. PMID: 10728704.
17. Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays. 1991; 13:31–36. PMID: 1722975.

18. Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003; 63:4342–4346. PMID: 12907602.
19. Furstenberger G, von Moos R, Lucas R, Thurlimann B, Senn HJ, Hamacher J, et al. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy or primary breast cancer. Br J Cancer. 2006; 94:524–531. PMID: 16450002.
20. Hudis CA. Clinical implications of antiangiogenic therapies. Oncology. 2005; 19:26–31. PMID: 15934500.
21. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275:964–967. PMID: 9020076.

22. Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis: implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005; 7:101–111. PMID: 15652753.
23. Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: from promiscuity to surrogate marker and target identification. Nature Rev Cancer. 2006; 6:835–845. PMID: 17036040.
24. Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003; 300:1155–1159. PMID: 12750523.

25. Spring H, Schuler T, Arnold B, Hammerling GJ, Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci USA. 2005; 102:18111–18116. PMID: 16326806.

26. Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006; 313:1785–1787. PMID: 16990548.

27. Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005; 5:423–435. PMID: 15928673.

28. Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4(+)CD25(+) regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2006; 56:641–648. PMID: 16960692.

29. Shaked Y, Emmengger U, Man S, Cervi D, Bertolini F, Ben-David Y, et al. The optimal biological dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005; 106:3058–3061. PMID: 15998832.
30. Ng SS, Sparreboom A, Shaked Y, Lee C, Man S, Desai N, et al. Influence of formulation vehicle on metronomic taxane chemotherapy: albumin-bound versus cremophor EL-based paclitaxel. Clin Cancer Res. 2006; 12:4331–4338. PMID: 16857808.

31. Munoz R, Man S, Shaked Y, Lee C, Wong J, Francia G, et al. Highly efficacious non-toxic treatment for advanced metastatic breast cancer using combination UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006; 66:3386–3391. PMID: 16585158.
32. Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, et al. Circulating endothelial cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006; 108:452–459. PMID: 16543470.
33. Man S, Bocci G, Francia G, Green S, Jothy S, Bergers G, et al. Antitumor and anti-angiogenic effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002; 62:2731–2735. PMID: 12019144.
34. Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived - but they can be improved. Cancer Biol Ther. 2003; 2:S134–S139. PMID: 14508091.
35. Man S, Munoz R, Kerbel RS. On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing. Cancer Metastasis Rev. 2007; epub ahead of print.

36. Rocca A, Dellapasqua S, Pietri E. Metronomic chemotherapy with capecitabine and oral cyclophosphamide in combination with bevacizumab in metastatic breast cancer (mbc): evidence of activity of an antianigogenic treatment. Proc Am Soc Clin Oncol. 2007; #11501(2007) (Abstract).
37. Buckstein R, Crump M, Shaked Y, Nayar R, Foden C, Turner R, et al. High dose celecoxib and metronomic 'low dose' cyclophosphamide is effective and safe therapy in patients with relapsed and refractory aggressive histology NHL. Clin Cancer Res. 2006; 12:5190–5198. PMID: 16951238.
38. Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low dose metronomic oral cyclophosphamide in recurrent ovarian cancer. A study of the California, Chicago and Princess Margaret Hospital Phase II Consortia. J Clin Oncol. 2007; in press.
39. Glode LM, Crighton F, Barqawi A, Kerbel RS, Berman C, Crawford D. Metronomic therapy with cyclophosphamide and dexamethasone for prostate cancer. Cancer. 2003; 98:1643–1648. PMID: 14534880.
40. Orlando L, Cardillo A, Rocca A, Balduzzi A, Ghisini R, Peruzzotti G, et al. Prolonged clinical benefit with metronomic chemotherapy in patients with metastatic breast cancer. Anticancer Drugs. 2006; 17:961–967. PMID: 16940806.

41. Vogt T, Hafner C, Bross K, Bataille F, Jauch KW, Berand A, et al. Antiangiogenetic therapy with pioglitazone, rofecoxib, and metronomic trofosfamide in patients with advanced malignant vascular tumors. Cancer. 2003; 98:2251–2256. PMID: 14601096.

42. Kong DS, Lee JI, Kim WS, Son MJ, Lim dH, Kim ST, et al. A pilot study of metronomic temozolomide treatment in patients with recurrent temozolomide-refractory glioblastoma. Oncol Rep. 2006; 16:1117–1121. PMID: 17016602.

43. Reichle A, Bross K, Vogt T, Bataille F, Wild P, Berand A, et al. Pioglitazone and rofecoxib combined with angiostatically scheduled trofosfamide in the treatment of far-advanced melanoma and soft tissue sarcoma. Cancer. 2004; 101:2247–2256. PMID: 15470711.

44. Buckstein R, Kerbel RS, Shaked Y, Nayar R, Foden C, Turner R, et al. High-dose celecoxib and metronomic "low-dose" cyclophosphamide is an effective and safe therapy in patients with relapsed and refractory aggressive histology non-Hodgkin's lymphoma. Clin Cancer Res. 2006; 12:5190–5198. PMID: 16951238.

45. Young SD, Whissell M, Noble JC, Cano PO, Lopez PG, Germond CJ. Phase II clinical trial results involving treatment with low-dose daily oral cyclophosphamide, weekly vinblastine, and rofecoxib in patients with advanced solid tumors. Clin Cancer Res. 2006; 12:3092–3098. PMID: 16707607.

Fig. 1
Differential effects of maximum tolerated dose cyclophosphamide (MTD CTX) versus metronomic low dose chemotherapy on tumor growth (upper panel) or levels of circulating endothelial progenitor cells (CEPs) in peripheral blood of mice (bottom panel). NOD-SCID mice were injected with a human (Granta) lymphoma cells, subcutaneously, and established tumors were then treated with three different forms of CTX, or left untreated. MTD CTX was given in successive 6-day cycles where the drug was injected at 150 mg/kg every other day (shorter vertical arrows) separated by 2-week break periods. The metronomic regimens consisted of either daily continuous administration through the drinking water at an approximate dose of 20 mg/kg per day or weekly intraperitoneal/bolus injections of the drug of 150 mg/kg. Note the far greater anti-tumor efficacy of the two metronomic regimens compared to the conventional and more toxic MTD regimen. The bottom panel illustrates the observation of increasing levels of CEPs in untreated tumor bearing mice with progressive increase in tumor volume. However, when tumor bearing mice are treated with a course of MTD CTX there is an initial decline in CEPs which is then followed by a marked rebound during the drug-free break periods. In contrast, the two different metronomic regimens caused a sustained suppression of CEPs throughout the course of therapy. Taken from Bertolini et al (18) and reproduced with permission of the authors.
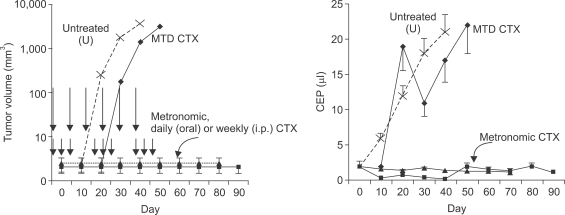
Fig. 2
Acute homing of bone marrow derived circulating cells (including endothelial progenitor cells) to tumors shortly after tumor bearing mice are treated with a single injection of a vascular disrupting agent (VDA) called OXi-4503. Lewis Lung carcinoma cells were grown in syngeneic C57/Bl6 mice that had previously been lethally irradiated and reconstituted with syngeneic GFP-positive bone marrow cells. Note low levels of GFP-positive cells in tumors from untreated (control) or DC101 treated mice. However, 72 hours after OXi-4503 treatment a pronounced GFP cell colonization is evident, a process which can be prevented by prior treatment with DC101, an anti-VEGFR-2 monoclonal neutralizing antibody. The CEP homing phenomenon was shown to contribute to tumor angiogenesis and tumor growth at the viable tumor rim which characteristically remains after VDA treatment. Taken from Shaked et al (26) with permission of the authors.
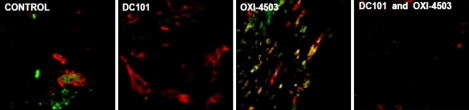
Fig. 3
Using circulating endothelial progenitor cells (CEPs) in peripheral blood as a surrogate biomarker for establishing the optimal biologic dose (OBD) for metronomic chemotherapy. Tumor bearing mice were treated with cyclophosphamide (CTX) daily for a week, or vinorelbine (Vbn) or vinblastine (Vbl) twice a week by intraperitoneal injection at the indicated doses and then viable CEPs assessed in peripheral blood samples by four color flow cytometry. In addition, anti-tumor effects and host toxicity (e.g. loss of body weight) were assessed in separate experiments, and correlated with the CEP results. The OBDs for the three drugs are highlighted in boxes, e.g. 20 mg/kg/day for CTX, 9 mg/kg/dose for Vbn and 0.33 mg/kg/dose for Vbl, which coincide with the nadir of CEPs detected and maximal anti-tumor activity along with acceptable or no toxicity. All tumors tested were of human origin, and were described in Shaked et al (29). Significant differences from control are present by *p>0.05, †0.05>p>0.01 and ‡p<0.01.
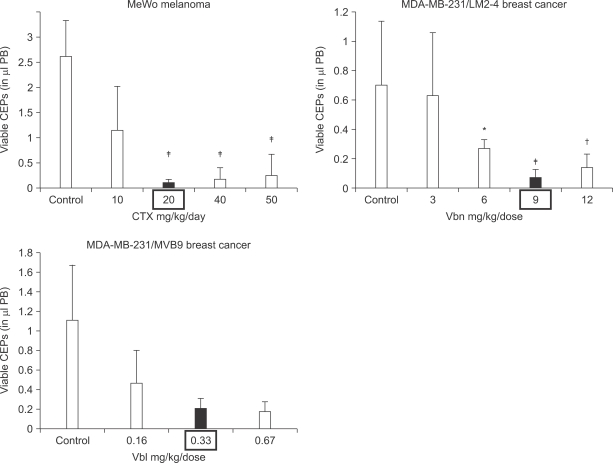
Fig. 4
Procedure used for selecting the highly metastatic MDA-MB-231 human breast cancer variant, 231/LM2-4. MDA-MB-231 cells were injected into the mammary fat pads (MFPs) of SB-17 SCID mice, i.e., orthotopically injected. The 231/LM2-4 variant was obtained subsequent to two rounds of lung metastasis selection in mice, after surgical removal of the primary orthotopically transplanted tumor, as explained in detail in the text and outlined in the diagram. Primary tumors were removed when volumes reached approximately 400 mm2. Six weeks later mice were sacrificed and examined for the presence of macrometastases in the lungs, liver, lymph nodes and MFPs. Blue arrows indicate typical location of metastatic lesions. The sequence for the selection strategy was as follows: i) 2×106 MDA-MB 231 cells were injected into the MFP of adult 6~8 week old female CB17 SCID mice; ii) four weeks later the primary tumor was resected fully by surgery when the tumor volumes were 300~500 mm3; iii) at monthly intervals, groups of mice were sacrificed and checked for presence of metastases in the lungs and full blown diffuse metastatic spread was observed in the mice after 4 months; iv) the whole set of lungs from one mouse were adapted to tissue culture and grown for 3 passages to derive a line referred to as 231/LM1; v) 2X106 231/LM1 cells were injected into the MFP of adult 6~8 week old female CB17 SCID mice; vi) approximately 3 weeks later the primary tumor was resected when the tumor volume was 300~500 mm3; vii) again, at monthly intervals, groups of mice were sacrificed and checked for metastases in the lungs; 2 months after resection of the primary tumor, mice were observed to have numerous macroscopic lung nodules with some spilling into the pleural cavity; viii) several individual lung nodules were isolated and adapted to tissue culture to derive established lines, one of which 231/LM2-4, was selected for in vivo studies. Overall, this selection procedure took almost nine months to complete. Genotypic analysis of MDA-MB-231 and the 231/LM2-4 variant verified their human origin and lineage relationship (data not shown). Surgical resection of primary tumors was carried out by skin incision and carefully clearing all tumor tissue away from surrounding connective tissue. Weekly weight assessment was used as a surrogate marker for toxicity. The mice were sacrificed when tumor sizes reached 1.7 cm3. The bottom panel shows a photograph of the pattern of metastatic spread that can be attained within one month using the LM2.4 variant after surgical excision of the primary tumor. Taken from Munoz et al (31) with permission of the authors.
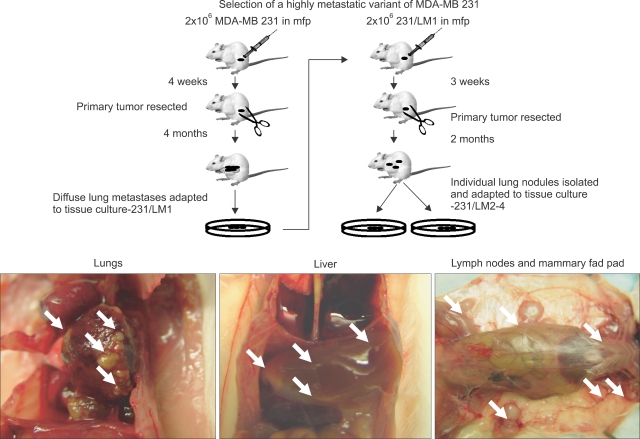
Fig. 5
Chronic combination oral metronomic low-dose CTX and UFT prolongs survival of mice with advanced metastatic disease. 231/LM2-4 human breast metastatic variant cells were orthotopically injected into the MFPs of 6~8 week old CB17 SCID mice. When tumors reached volumes of approximately 200 mm3, treatment with either vehicle control, or 15 mg/kg/day UFT by gavage, or 20 mg/kg/day CTX through the drinking water, or a combination of CTX and UFT treatments was initiated. Tumors were measured weekly and tumor volume was plotted accordingly. Arrow indicates time of initiation of treatment. When tumors reached 400 mm3 (which took approximately 3 weeks) primary tumors were surgically removed. Treatment with vehicle control, 15 mg/kg/day UFT by gavage, 20 mg/kg/day CTX through the drinking water, or the daily combination of metronomic UFT and CTX, were initiated 3 weeks after surgery on a daily non-stop basis. For example, the duration of the therapy was 140 days, and was initiated on day 43, 3 weeks after surgery, with termination at day 183. Mice were monitored frequently according to the institutional guidelines. A Kaplan-Meier survival curve was plotted accordingly for all treated group, as indicated in the figure. n=7~9 mice/group. Taken from Munoz et al (31) with permission of the authors.
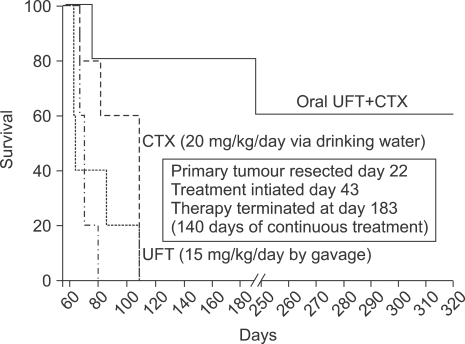
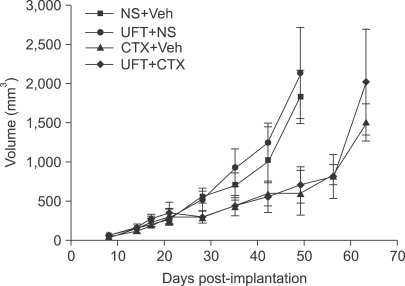
Fig. 6
Effect of metronomic cyclophosphamide (CTX) or metronomic UFT, or the two drugs together on the growth of orthotopically implanted MDA-MB-231 LM2.4 human breast cancer carcinoma. Treatment was started when tumors in the mammary fat pad attained a size of about 200 mm3 and was maintained until mice had to be sacrificed. The doses and schedules used are as for Fig. 5. NS: normal saline, Veh: vehicle control for UFT. Note lack of effect of UFT alone on primary tumor volumes or when added to metronomic CTX. However, metronomic UFT was found to have a potent local anti-invasive effect on the primary tumors and also inhibited development of microscopic metastases as described by Munoz et al (31). Taken from Munoz et al (31) with permission of the authors.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download