Abstract
Purpose
We wanted to determine and report on the outcome of combined gemcitabine/cisplatin chemotherapy for patients suffering with locally advanced or metastatic urothelial cancer.
Materials and Methods
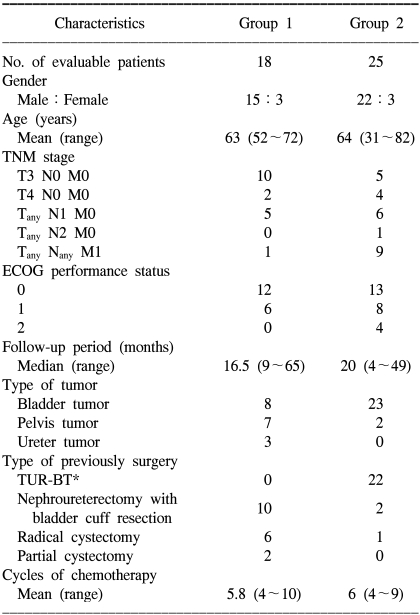
Between July 1999 and December 2004, 43 selected patients were enrolled in this study. Group 1 (the adjuvant chemotherapy group) had undergone radical surgery with removal of evident tumor from the following primary sites: bladder (n=8), renal pelvis (n=7) and ureter (n=3). Group 2 (the salvage chemotherapy group) had undergone palliative surgery with a remnant tumor at the following primary sites; bladder (n=23) and renal pelvis (n=2). All the patients were given gemcitabine/ciplatin and they evaluated for the therapeutic effect and toxicity. The patients were initially treated with gemcitabine 1000 mg/m2 intravenously for 30 minutes on days 1, 8 and 15 of a 28-day cycle, and cisplatin 70 mg/m2 was administered intravenously on day 1 using prehydration measures.
Results
Group 1: The median follow-up period was 16.5 months. The mean age was 63 years (males: 15 cases, females: 3 cases), and eleven patients (61%) remained alive. The estimated median relapse-free survival period and 2-year survival rate were 24 months and 63%, respectively. Group 2: the median follow-up period was 20 months, the mean patient age was 63.8 years (males: 22 cases, females: 3 cases), and nine patients (36%) remained alive. The overall response and 2-year survival rates were 36% and 43%, respectively. Toxicities: Grade 3 toxicities developed in 14 cycles during the total 232 cycles. Grade 4 toxicity did not occur.
Transitional cell carcinoma (TCC) of the urothelium is a common cancer worldwide and the incidence of this cancer is increasing. Before the development of effective chemotherapy, the median survival rarely exceeded 3 to 6 months for advanced urothelial TCC (1). However, after the efficacy of a combination chemotherapy based on methotrexate, vinblastin, adriamycin and cisplatin (MVAC) for treating metastatic urothelial cancer was first described in 1985 (2), MVAC became the standard treatment for advanced urothelial cancer. In the Phase III studies of MVAC therapy for patients with advanced urothelial TCC, the overall response rates were found to be 40~70% and median survival period was approximately 12 months (3~5). However, in a recent long-term follow-up study, only 3.7% of the patients randomized to MVAC remained continuously disease free at 6 years (6). In addition, severe adverse effects such as drug related death, granulocytopenic fever, sepsis and mucositis have been associated with the MVAC regimen.
For these reasons, more effective and less toxic drugs are required; several new agents and combination regimens have demonstrated activity against urothelial cancer. These agents include gemcitabine, the taxanes, carboplatin and ifospamide. Thus, the incorporation of these agents into new chemotherapeutic combinations and also modification of the MVAC regimen have been investigated in order to improve the results and ameliorate the toxicity of the MVAC regimen (7~9).
The current study was designed to evaluate the safety and efficacy of combined gemcitabine/cisplatin chemotherapy (GC) for patients with locally advanced or metastatic urothelial cancer. Moreover, all the previous reports of administering gemcitabine for TCC of the urothelium have underscored its high activity and low toxicity, thus indicating that this agent in combination with cisplatin merits further investigation (10).
Patients with histologically proven advanced TCC of the urinary tract and who were treated at the Kyung Hee University and Cheju University Medical Center were enrolled into this study. The exclusion criteria were previous radiotherapy and/or chemotherapy, the presence of another cancer or a serious concomitant systemic disorder. The patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. The patients were divided into two groups. Group 1 was the adjuvant chemotherapy group, which was comprised of patients who were at high risk of locoregional relapse after radical surgery. This group included patients with locally advanced stage tumor such as T3 or T4a without any nodal and distal metastasis. Group 2 was the salvage chemotherapy group that was comprised of patients who had received palliative surgery and they had remnant tumor, lymph node metastasis or distant metastasis.
Evaluation of disease in all cases included a bone scan, chest x-ray and computerized tomography or magnetic resonance imaging (MRI).
Before the initiation of chemotherapy, all the patients underwent a complete medical history, a physical examination, a performance status evaluation, a complete blood cell count, routine serum chemistry studies and urinalysis and creatinine clearance (CrCl) testing; these tests were repeated prior to each cycle. Complete blood counts with biochemical assessment were performed after 8 and 15 days of gemcitabine administration.
We started chemotherapy 14 days after the patients' operations. The patients were initially treated with gemcitabine 1,000 mg/m2 intravenously for 30 minutes on days 1, 8 and 15 of a 28-day cycle, and cisplatin 70 mg/m2 was administered intravenously on day 1 with using prehydration measures.
Treatment was delayed in the patients with a granulocyte count of <1,500 cells/mm3 or a platelet count of <75,000 cells/mm3 or non-hematologic toxicity above grade 3. The maximal delayed time was 2 weeks and if more time was needed, then the patient was dropped from the study. Granulocyte-colony stimulating factor was administered when leukocytopenia developed (neutrophil count <1,000/mm3). A red blood cell transfusion was administered when anemia developed (hemoglobin <9.0 g/dl). If the CrCl was lower than 40 mg/min, then the dosage of cisplatin was reduced by 30%. For other non-hematologic toxicity above grade 3, administration of the anti-cancer medicine was delayed until the toxicity decreased to less than grade 2.
We initially planed 6 treatment cycles in both group and we planed additional 3 cycles for the patients who tolerated the chemo regimen and were responsive to it.
Radiographic analyses of tumor size, tumor burden and disease staging were carried out at baseline and after every fourth cycle or as indicated clinically.
The responses to GC chemotherapy were determined using the following definitions.
A relapse-free response was defined as no evidence of disease recurrence for any clinically detectable disease for a minimum of 4 weeks, i.e., no new lesions, no evidence of nonevaluable disease and no disease-related symptoms.
Because of the absence of any evaluable lesion after surgery in group 1, we decided upon assessing the response to treatment using relapse-free survival and overall survival.
The relapse-free survival and overall survival durations were recorded from the date of surgery to the date of documented relapse or death, respectively. The patients who had not relapsed or were alive with/without disease were censored from the relevant analysis.
The Kaplan-Meier method was used to analyze the relapse free survival and overall survival with using 95% confidence intervals (CI).
The patients were evaluated according to the WHO criteria (11). A complete response (CR) was defined as the complete disappearance of all clinically detectable disease for a minimum of 4 weeks and there was no development of new lesions. A partial response (PR) was defined as a 50% reduction in the sum of the products of the two greatest perpendicular dimensions of all measurable lesions for at least 4 weeks with no simultaneous progression of evaluable disease or the appearance of new lesions. Stable disease (SD) was defined as an increase of <25% in the sum of the products of the two greatest perpendicular dimensions of all measurable lesions and the absence of a partial or complete response. Progressive disease (PD) was defined as an increase of 25% or an increase of 10 cm2 in the sum of the products of the 2 longest perpendicular dimensions of each measurable lesion or a clear worsening of any evaluable disease or the appearance of any new lesion or the patient's failure to return to the clinic due to deteriorating disease (unless the deterioration clearly was unrelated to disease progression). Stable and progressive disease were both considered treatment failure. We planned to continuously perform chemotherapy until there was a response if the patients tolerated the treatment and it was possible.
The relapse free survival was determined from the date of the observed response until disease progression or until the last contact with the patient. The survival duration was measured from the date of initial treatment with GC until death, or until the last contact with the patient. If a patient discontinued treatments for any reason, he/she was contacted at monthly intervals to determine the survival duration. The progression free survival period was defined as the duration from the start date of GC chemotherapy to disease progression or to discontinuation due to death or drug-related toxicities. The Kaplan-Meier method was used to analyze the response duration, the time to progression and the overall survival with using 95% CIs.
Between October 1999 and April 2005, 43 selected patients were enrolled in this study. Of these 43 study subjects, 95% of them had an ECOG performance status of 0 or 1. The mean age of groups 1 and 2 were 63 years (range: 52~72 years) and 64 years (range: 31~82 years), respectively. The primary cancer site was the urinary bladder in 31 patients and the upper urinary tract in 12 patients (the renal pelvis in 9 and the ureter in 3 patients). The previous surgeries among the study subjects included transurethral resection of bladder tumor, nephroureterectomy with bladder cuff resection, radical cystectomy and partial cystectomy. A total of 232 cycles were administrated. The detailed patient characteristics are presented in Table 1.
The mean number of cycle was 5.8 (range: 4~10 cycles). The variations in the number of treatment cycles were caused by discontinuation of treatment due to disease progression at any time, patient refusal or a patient being considered by the investigators to be unfit to continued treatment. The median follow-up period was 16.5 months (range: 9~65 months).
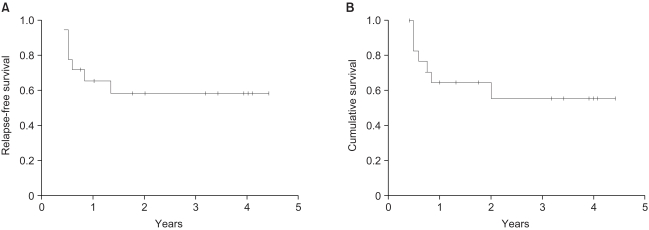
10 patients (56%) relapsed. The median relapse-free survival time was 24 months (95% CI: 0~56 months) and the relapse-free survival rate at 2 years was 46% (Fig. 1A). The overall 2-year survival rate for all the patients was 63% (Fig. 1B). At the time of analysis, 7 of the 18 patients had died, and all of them had relapsed.
The mean number of cycles was 6 (range: 4~9 cycles), and the median follow-up period was 20 months (range 4~49 months). 6 patients (24%) achieved CR, and 3 patients (12%) achieved PR for an overall response rate of 36% (95% CI: 20.3~55.5%). 2 patients (8%) achieved SD, and 14 (56%) patients experienced progressive disease. The median response duration was 18 months (95% CI: 11~25 months) and the progression free survival rate at 2 years was 36% (Fig. 2A). The overall median survival time and the 2-year survival rate in group 2 were 20 months (95% CI: 11~29 months) and 43%, respectively (Fig. 2B). At the time of censoring, 16 of the 25 patients in group 2 had died; 1 had CR, 13 had PD and 2 had PR. The one patient with CR died from non-disease related pneumonia.
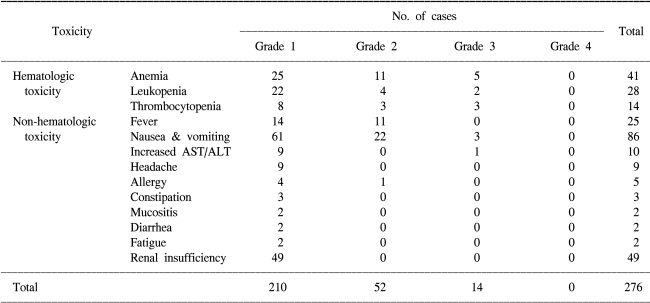
Toxicity was analyzed using the WHO toxicity criteria scale. As shown in Table 2, the toxicity pattern was generally tolerable, but some of the patients stopped treatment due to several reasons such as severe bone marrow toxicity, follow up loss and progressive disease or the development of recurrence.
The cisplatin dose was decreased in 49 cycles of the 232 total cycles (21%) because of renal insufficiency. Gemcitabine administration was delayed in 28 (12%) cycles because of hematologic toxicity. No toxic-related death occurred among the 43 study subjects. Only 16 cycles (7%) required administering granulocyte-colony stimulating factor for treating leucopenia (neutrophil count <1,000/mm3). Red blood cell transfusions were administered in 4 patients due to anemia (hemoglobin <9.0 g/dl). Nausea and vomiting were the most common side effects: this was grade 1~2 in 83 cycles and grade 3~4 in 3 cycles of the total 232 cycles. The other non-hematologic toxicity above grade 3 was an abnormality on a liver function test. Treatment was delayed for 2 weeks and that patient received the scheduled chemotherapy after the toxicity was decreased to less than grade 2.
Muscle-invasive urothelial cancer has a high probability of occult nodal and systemic micrometastasis at the time of diagnosis, and because the optimal time to treat these occult deposits is when the disease volume is minimal, chemotherapy regimens have increasingly been investigated in combination with definitive local therapy for treating the patients suffering with muscle-invasive urothelial cancer. Thus, adjuvant chemotherapy has been studied for the treatment of several solid tumors, e.g., breast cancer, pancreatic cancer and non-small cell lung cancer (12~14). In all cases, adjuvant therapy has been shown to increase the disease-free and overall survival times.
The advantages of adjuvant chemotherapy are that a specimen is available for pathologic evaluation, the prognostic factors for relapse and/or the development of metastases can be assessed, it eliminates the risk of second primary tumors and it causes no delays in the administration of the definitive treatment. The problem of adjuvant chemotherapy are that the absence of a marker lesion prevents assessment of chemosensitivity, the removal of the native organ, the treatment of micrometastases is delayed and there are difficulties in administering the planned chemotherapy at the correct dose-intensity. (15,16) However, despite these disadvantages, adjuvant chemotherapy has been widely adopted due to its benefits.
Suzuki et al, (17) have randomly compared radical surgery (i.e., radical cystectomy and radical nephroureterectomy), regional lymphadenectomy plus adjuvant chemotherapy (MVAC or MEC: methotrexate, epirubicin and cisplatin) and radical cystectomy alone for treating high-risk urothelial cancer. In the adjuvant chemotherapy group, the disease free survival and overall survival rates were 56% and 63% at 3 years, respectively, whereas the disease free survival and overall survival rates of the surgery only group were 10% and 27%, respectively. This trial demonstrated a significant disease-free survival advantage for the adjuvant treatment arm 3 years after radical surgery (p=0.051). In addition, a slight, although non-significant difference in overall survival was observed between the two groups (p=0.257).
Segal et al (15), after systematically reviewing the published literature, found five randomized controlled trials (RCTs) that examined adjuvant chemotherapies. These trials also failed to prove a benefit in term of overall survival, but they did suggest that adjuvant chemotherapy may benefit patients with advanced TCC. In addition to these studies, our data also shows that the relapse-free survival and overall survival rates were 46% and 63% in group 1, respectively. Although our stuidy was unlike other studies as we did not compare adjuvant chemotherapy with surgery alone, our data show a positive impact on the survival of group 1 patients.
von der Masse et al (18), have compared efficacies of GC and MVAC for treating advanced or metastatic bladder cancer patients, and no difference was found between the two in terms of survival or the interval to progression.
Urothelial cancer has been a chemosensitive tumor since the advent of MVAC; nevertheless metastatic disease remains essentially fatal and only a small number of patients achieve long-term disease control. In addition, the long term follow-up of MVAC showed that the side effects of MVAC are a serious problem. Hence, new chemotherapeutic agents and combinations of these agents are required to treat urothelial cancer. A number of studies using GC for treating urothelial cancer have been done. Kaufman et al (19), reported an overall response rate of 41% (CR: 22%, PR: 19%), a median time to treatment failure of 5.5 months and a median overall survival of 14.3 months for the metastatic urothelial cancer patients who were treated with GC. Moore et al (20), reported that using GC for advanced urothelial cancer produced an overall response rate and a median survival of 57% (CR: 21%, PR: 36%) and 13.2 months, respectively, which concurs with our findings of an overall response rate and a median survival of 36% (CR: 24%, PR: 12%) and 20 months, respectively.
A recent comparative trial of GC versus MVAC for treating advanced urothelial cancer patients also reported similar overall response rates (GC: 54%, MVAC: 53%) and median survival (GC: 15.4 months, MVAC: 16.1 months) (21).
The majority of urothelial cancer patients are elderly people, and many patients also have pulmonary and/or cardiovascular disease due to smoking. This increase in the numbers of elderly patients has altered the demographics of cancer and this highlights the need to develop more age-appropriate treatment protocols. The problems of systemic chemotherapy for elderly urothelial cancer patients are morbidity and tolerability. MVAC causes significant clinical myelosuppression with up to a 25% incidence of granulocytopenic fever at any time during the course of MVAC therapy; there is also up to a 50% incidence of grade 2~3 mucositis and 3% incidence of drug-related mortality (22).
Hence, regimens that are less toxic than MVAC such as the GC regimen have been developed during the past decade, and they appear to have approximately the same efficacy as MVAC against metastatic urothelial cancer. The good safety profile of gemcitabine, its proven in vitro synergism with other anticancer drugs and its lack of overlapping toxicity with cisplatin make GC an attractive combination as compared with the reference regimens.
Meliani et al (23) have evaluated the GC toxicities in 40 invasive bladder cancer patients. Seven of 40 (17.5%) patients developed grade 2 anemia; 19/40 (47.5%) developed thrombocytopenia [10 with grade 1, 8 with grade 2 and 1 with grade 4 (2.5%)]; 12/40 (30%) developed granulocytopenia (5 with grade 1, 4 with grade 2 and 3 with grade 3), and 7/40 (17.5%) developed grade 2 fever associated with leukopenia. There weren't any grade 3~4 non-hematololgic toxicities related to drug administration. The most common non-hematologic toxicities were constipation or diarrhea in 10/40 cases (25%). Acute nausea and vomiting were seen in only 1 patient. The most common hematological toxicity was anemia (17%), and this was followed by leucopenia and thrombocytopenia. The main difference between Meliani's findings and our findings was concerned with non-hematologic toxicities; in our study nausea and vomiting were the most common non-hematologic toxicity.
Lehmann et al (21), compared the toxicities of GC and MVAC in advanced urothelial carcinoma patients. The toxic death rate was 0% in the GC arm and 3% in the MVAC arm. Significantly more GC patients than MVAC patients experienced grade 3/4 anemia (GC patients: 52%, MVAC patients: 20%) and significantly more red blood cell transfusions were required in the GC arm. Significantly more GC patients than MVAC patients had grade 3/4 thrombocytopenia (GC patients: 54%, MVAC patients: 17%) without grade 3/4 hemorrhage or hematuria in either arm. In addition, more MVAC patients experienced grade 3/4 neutropenia (GC patients: 56%, MVAC patients: 61%, p=1.000), neutropenic or leukopenic fever (GC patients: 0%, MVAC patients: 10%, p=0.237), mucositis (GC patients: 0%, MVAC patients: 7%, p=0.495), and alopecia (GC patients: 6%, MVAC patients: 36%, p=0.004). Although anemia and thrombocytopenia were significantly more common in the GC arm than the MVAC arm, the grades of the toxicity in the GC arm were lower.
The limitation of the present study is that the better safety and tolerability of GC was not reflected in the quality of life results. However, GC was found to be a significant safer therapeutic regimen for treating advanced urothelial cancer patients.
References
1. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000; 18:3068–3077. PMID: 11001674.

2. Sternberg CN, Yagoda A, Scher HI, Watson RC, Ahmed T, Weiselberg LR, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol. 1985; 133:403–407. PMID: 4038749.

3. McCaffrey JA, Dodd PM, Herr H, Vlamis V, Mazumdar M, Higgins G, et al. Nonbladder primary site of transitional cell carcinoma (TCC) does not affect probability of response to M-VAC or survival (abstract 1299). Proc Am Soc Clin Oncol. 1998; 17:337a.
4. Sternberg CN, Yagoda A, Scher HI, Watson RC, Geller N, Herr HW, et al. M-VAC for advanced transitional cell carcinoma of the urothelium: efficacy, and patterns of response and relapse. Cancer. 1989; 64:2448–2458. PMID: 2819654.
5. Tannock I, Gospodarowicz M, Connolly J, Jewett M. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) chemotherapy for transitional cell carcinoma: the Princess Margaret Hospital experience. J Urol. 1989; 142(2 Pt 1):289–292. PMID: 2746745.

6. Saxman SB, Propert KJ, Einhorn LH, Crawford ED, Tannock I, Raghavan D, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1997; 15:2564–2569. PMID: 9215826.

7. Gitlitz BJ, Baker C, Chapman Y, Allen HJ, Bosserman LD, Patel R, et al. A phase II study of gemcitabine and docetaxel therapy in patients with advanced urothelial carcinoma. Cancer. 2003; 98:1863–1869. PMID: 14584068.

8. Calabro F, Sternberg CN. New drugs and new approaches for the treatment of metastatic urothelial cancer. World J Urol. 2002; 20:158–166. PMID: 12196899.

9. Bellmunt J, Albiol S. New chemotherapy combinations for advanced bladder cancer. Curr Opin Urol. 2001; 11:517–522. PMID: 11493774.

10. Lorusso V, Manzione L, De Vita F, Antimi M, Selvaggi FP, De Lena M. Gemcitabine plus cisplatin for advanced transitional cell carcinoma of the urinary tract: a phase II multicenter trial. J Urol. 2000; 164:53–56. PMID: 10840423.

11. Perry MC, Anderson CM, Donehower RC. Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, Mckenna WG, editors. Chemotherapy. Clinical oncology. 2004. New York: Churchill Livingstone;p. 492–493.
12. Burch PA, Mailliard JA, Hillman DW, Perez EA, Krook JE, Rowland KM, et al. Phase II study of gemcitabine plus cisplatin in patients with metastatic breast cancer: a North Central Cancer Treatment Group Trial. Am J Clin Oncol. 2005; 28:195–200. PMID: 15803016.
13. Wilkowski R, Thoma M, Duhmke E, Rau HG, Heinemann V. Concurrent chemoradiotherapy with gemcitabine and cisplatin after incomplete (R1) resection of locally advanced pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2004; 58:768–772. PMID: 14967432.

14. Byrne MJ, Phillips M, Powell A, Cameron F, Joseph D, Spry N, et al. Cisplatin and gemcitabine induction chemotherapy followed by concurrent chemoradiotherapy or surgery for locally advanced non-small cell lung cancer. Intern Med J. 2005; 35:336–342. PMID: 15892762.

15. Segal R, Winquist E, Lukka H, Chin JL, Brundage M, Markman BR. Cancer Care Ontario Practice Guidelines Initiative Genitourinary Cancer Disease Site Group. Adjuvant chemotherapy for deep muscle-invasive transitional cell bladder carcinoma - a practice guideline. Can J Urol. 2002; 9:1625–1633. PMID: 12431323.
16. Juffs HG, Moore MJ, Tannock IF. The role of systemic chemotherapy in the management of muscle-invasive bladder cancer. Lancet Oncol. 2002; 3:738–747. PMID: 12473515.

17. Suzuki S, Shinohara N, Harabayashi T, Sato S, Abe T, Koyanagi T. Impact of adjuvant systemic chemotherapy on postoperative survival in patients with high-risk urothelial cancer. Int J Urol. 2004; 11:456–460. PMID: 15242352.

18. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000; 18:3068–3077. PMID: 11001674.

19. Kaufman D, Raghavan D, Carducci M, Levine EG, Murphy B, Aisner J, et al. Phase II trial of gemcitabine plus cisplatin in patients with metastatic urothelial cancer. J Clin Oncol. 2000; 18:1921–1927. PMID: 10784633.

20. Moore MJ, Winquist EW, Murray N, Tannock IF, Huan S, Bennett K, et al. Gemcitabine plus cisplatin, an active regimen in advanced urothelial cancer: a phase II trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1999; 17:2876–2881. PMID: 10561365.

21. Lehmann J, Retz M, Steiner G, Albers P, Jaeger E, Knuth A, et al. Gemcitabine/cisplatin vs MVAC 5 year survival outcome of the phase III study of chemotherapy of advanced urothelial carcinoma in Germany. Urologe A. 2003; 42:1074–1086. PMID: 14513232.
22. de Wit R, Bellmunt J. Overview of gemcitabine triplets in metastatic bladder cancer. Crit Rev Oncol Hematol. 2003; 45:191–197. PMID: 12604129.
23. Meliani E, Lapini A, Serni S, Corvino C, Carini M. Gemcitabine plus cisplatin in adjuvant regimen for bladder cancer. Toxicity evalu ation. Urol Int. 2003; 71:37–40. PMID: 12845258.
Fig. 1
(A) Relapse-free survival curve after surgery for the patients with high risk urothelial cancer (group 1). (B) Overall survival curve after surgery for patients with high risk urothelial cancer (group 1).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download