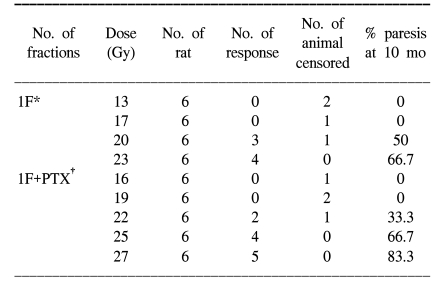
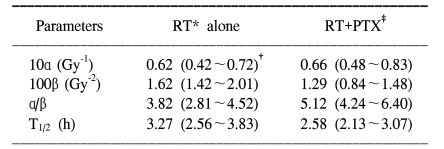
Adult female Sprague-Dawley rats (7 weeks old, median weight: 250 g) were used in this study. Two study groups were defined. The experimental group was treated with PTX in drinking water starting at 1 week prior to radiation, and this continued through 40 weeks after the radiation treatment. The control group received sterile drinking water without PTX. Pentoxifylline (PTX, Handok pharmaceutical Co., Seoul, Korea) was added to the distilled drinking water at a concentration of 2 g/L; the drinking water was consumed ad libitum. A 2 cm segment of the cervical spinal cord (C1 through T2) was irradiated with a 6MV LINAC at a dose rate of 3 Gy/min. The animals were irradiated with single, two, four and eight fractions with a fraction interval of 24 h. Each fractionation scheme was comprised four or five dose levels and there were 6 animals used for each dose level. For the control animals, the total doses ranged from 13 to 23 Gy, 27 to 37 Gy, 36 to 48 Gy and 44 to 54 Gy for the single, two, four and eight fractions, respectively. The corresponding total doses for the PTX groups ranged from 16 to 27 Gy, 30 to 41 Gy, 40 to 53 Gy and 48 to 59 Gy, respectively. The endpoint of the experiment was leg paresis and follow-up of the animals was continued for up to 40 weeks after the last irradiation. If we assume that the observed paralytic effects of cervical irradiation are a consequence of equivalent levels of cell lethality (for example, this was chosen for illustrative purposes to be equal to e
-1), then α/β can be calculated via reciprocal-dose analysis (
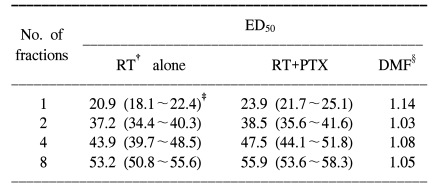
6). After tabulation of the ED
50 values (the estimated dose needed to produce 50% paralysis in a group of irradiated animals) with using the Litchfield and Wilcoxon method (
7), plotting the reciprocal of each ED
50 against the corresponding dose per fraction (=ED
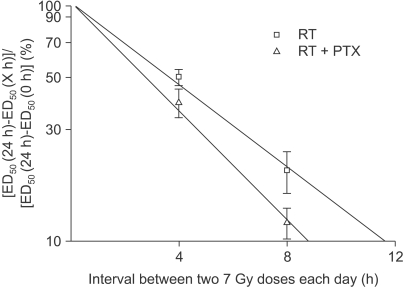
50/n) and performing linear least square regression resulted in a straight line. The intercept on the ordinate gave α, while the slope gave β. Subsequently, to obtain the relevant data on repair kinetics, the animals in the experimental group received a pair of 7 Gy fractions on each day, separated by intervals of 4 and 8 h. The repair half time was analyzed by the traditional process (
8). The ED
50 values were plotted linearly against the interval between the two 7 Gy doses on each day to show the pattern of repair. The ED
50 at the 24 h interval (7 Gy-24 h-7 Gy) was taken as corresponding to complete 100% repair and the ED
50 at the 0 h interval (7 Gy-0 h-7 Gy) as corresponding to zero repair. Making the semilog plot required an estimate of ED
50 for the 24 h interval, that is, with a 7 Gy dose given daily and the ED
50 for the zero interval, that is, with a 14 Gy dose given daily. This dose-response curve was not obtained from the present set of experiments and these ED
50 values were extrapolated from the existing data (
Fig. 1) with assuming that 7 or 14 Gy fractions had been given daily instead of the doses that were actually used. From this, the estimated ED
50 for the 7 and 14 Gy were 57.1 and 34.6 Gy for RT alone, respectively, and 63.3 Gy and 40.3 Gy for RT+PTX. The difference between the ED
50 at any other time interval and the ED
50 at 24 h was taken as a measure of the incomplete repair and this was plotted logarithmically as a percentage of the difference between the 24 h and the zero time ED
50 against the linear time interval. The inverse slope (ln2/slope) of the resulting regression line would give the T
1/2 if the incomplete-repair model with monoexponential kinetics was appropriate (
8). The detailed description of the materials and methods we used has been reported in our previous paper (
5). The significance of differences was tested using the t-test.
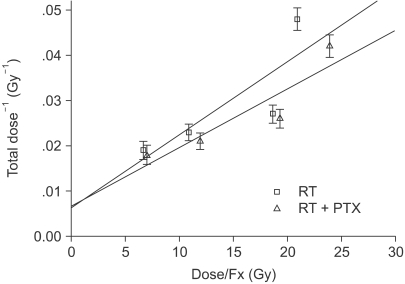 | Fig. 1Plot of the reciprocal total dose versus the dose per fraction needed to produce 50% paralysis in rats after irradiation of the cervical region of the spinal cord (RT: radiation, PTX: pentoxifylline). 
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download