Abstract
Purpose
Obesity-related leptin and leptin receptor (OBR) have a relation to the development of cancer and metastasis and also the low survival rate for breast cancer patients. Leptin has been associated with increased aromatase activity and it displays functional cross-talk with estrogen. This study was designed to determine the relationship between the expression of leptin and OBR in breast cancer tissue and the prognosis of early-stage breast cancer patients, and especially for the tamoxifen-treated patients.
Materials and Methods
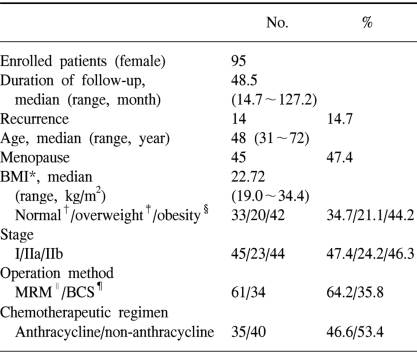
Ninety-five patients with early-stage breast cancer and who had undergone surgical treatment at Kyung Hee University Hospital between January 1994 and June 2004 were analyzed. The surgical specimens underwent immunohistochemical analysis for leptin and OBR. The patients' survival and clinical characteristics were obtained from the medical records.
Results
Of the 95 patients, 79 (83%) and 32 (33.7%) showed the expression of leptin and OBR in breast cancer tissue, respectively. The expression of leptin and OBR in breast cancer tissue was not significantly related to the clinicopathological characteristics, including obesity, the expression of hormonal receptor, the HER-2/neu expression, menopause, stage and the nuclear grade. The expression of leptin and OBR was not significantly related to the overall disease-free survival (DFS). For the tamoxifen-treated postmenopausal obese patients, the DFS of the leptin-positive group was higher than that of the leptin-negative group (p=0.017).
Conclusion
The expression of leptin and OBR in breast cancer tissue may be not a prognostic factor for disease-free survival of breast cancer patients. In the future, further studies are needed to determine whether leptin expression could be a predictive factor for tamoxifen therapy in the postmenopausal obese subgroup among the early breast cancer patients.
With the changes of modern life style, the incidence of breast cancer now ranks as the number one cancer for Korean females. Among the risk factors for breast cancer, obesity has been focused on and especially for postmenopausal women (1). Obesity in postmenopausal women increases the estrogen level through activation of aromatase and thereafter the women may develop breast cancer (2).
Leptin is an adipocyte-derived cytokine, and it is an important mediator of obesity. Leptin is secreted from normal or malignant breast tissue as well as from adipose tissue, and it's involved in carcinogenesis of breast tissue and in the proliferation and angiogenesis of breast cancer cells (3). Leptin also has a significant association with the progression, distant metastasis and poor survival of breast cancer (4,5). The function of leptin is mediated by leptin receptor (OBR) and the role of OBR has been reported to be similar to that of leptin (6-8). It is expected that a new treatment modality for breast cancer could come about through functional and structural inhibition of the leptin system. This system might be helpful to create strategies to help women survive breast cancer.
Leptin and estrogen have a functional cross-talk relationship. Leptin induces the expression of aromatase genes in breast cancer cell lines and in adipose tissue, with a subsequent increase in the local estrogen production (3). Leptin also increases the expression of estrogen receptor, and it activates estrogen receptor via mitogen-activated protein kinase (MAPK) (4). Estrogen also controls the expression of leptin genes in the adipose tissue, and especially estradiol (E2), which acts on estrogen-responsive elements that are located at the promoter sites of the obesity genes (Ob genes) is a potent leptin secretor (5,9). Although it was reported that antiestrogen treatment increased the serum leptin level and leptin showed antiestrogen-resistance, the effect of leptin on antiestrogen may be controversial.
This study was designed to observe the clinicopathological characteristics of leptin-expressing and OBR-expressing early breast cancer. Further, we wanted to investigate the role of leptin and OBR as prognostic factors for the disease-free survival of early breast cancer patients and also as predictive markers for tamoxifen-treated early breast cancer patients.
This study included 115 patients with early-stage, invasive breast cancer; these patients received surgical treatment at the Kyung Hee University Hospital between January 1994 and June 2004. The following 20 patients were excluded from the study: 7 patients who did not receive >80% of their planned chemotherapy, 12 patients who were lost to follow-up for more than at least 1 year, 1 patient who did not receive tamoxifen therapy although her hormonal receptor status had been positive. The study subjects were limited to patients with stages I, IIa and IIb disease, and only patients with invasive breast cancer were analyzed to exclude any histological differences. The staging of the lesion was performed according to the TNM staging system proposed by the 2002 American Joint Committee on Cancer (AJCC) staging classification for breast cancer (10). Sixty-one patients received modified radical mastectomy and 34 patients received breast conserving surgery. If needed, chemotherapy or hormonal therapy was administered following surgery (Table 1). Forty patients received a chemotherapy regimen that consisted of cyclophosphamide, methotrexate and 5-fluorouracil (CMF): cyclophosphamide 100 mg/m2/day (day 1 through day 14) was orally administered, and the methotrexate 40 mg/m2/day (on days 1 and 8) and 5-fluorouracil 600 mg/m2/day (on days 1 and 8) were intravenously administered. This regimen was repeated at 4-week intervals thereafter. A total of 6 cycles of CMF chemotherapy were performed, and the patients who received >4 cycles were analyzed in this study. Two patients received a chemotherapy regimen that consisted of cyclophosphamide, doxorubicin and 5-fluorouracil (CAF): cyclophosphamide 600 mg/m2/day, doxorubicin 60 mg/m2/day and 5-fluorouracil 600 mg/m2/day were intravenously administered on day 1, and thereafter these drugs were repeated 4 times at 3-week intervals. Thirty-three patients received a chemotherapy regimen that consisted of doxorubicin and cyclophosphamide (AC): doxorubicin 60 mg/m2/day and cyclophosphamide 600 mg/m2/day were intravenously administered on day 1 and thereafter they were repeated 4 times at 3-week intervals. One patient received 5 cycles of neoadjuvant CEF chemotherapy preoperatively and then 4 cycles of CMF chemotherapy postoperatively. The CEF chemotherapy (cyclophosphamide 500 mg/m2/day, epirubicin 50 mg/m2/day and 5-fluorouracil 500 mg/m2/day) was given on day 1, and thereafter this was repeated at 3-week intervals. The patients who were positive for estrogen or progesterone receptor were defined as hormone receptor-positive, and all of these patients received maximum tamoxifen therapy for 5 years.
The tumor tissue specimens were immunohistochemically stained for leptin, OBR, estrogen and progesterone receptors and HER-2/neu. After light-microscopic examination of the samples from the 95 patients with invasive breast cancer and who received surgical treatment, the representative paraffin-embedded tissues that contained invasive cancer, normal mammary gland and normal adipose tissue were selected. All of the hematoxylin and eosin-stained slides were reviewed. For immunohistochemical staining of leptin and OBR, we used the streptavidin-biotin-peroxidase method by using a LSAB kit (DAKO, Capenteria, CA). Briefly, the slides were reacted with normal horse serum that was diluted with buffered solution at 1:10, and the non-specific material in the tissue was removed. The working dilutions and sources of the primary antibodies used in this study included rabbit polyclonal Ob antibody A-20 (Santa Cruz Biotechnology Inc., Santa Cruz, CA) at 1:200, mouse monoclonal Ob-R antibody B-3 (Santa Cruz Biotechnology Inc.) at 1:200, rabbit monoclonal c-erbB-2 antibody (DAKO) at 1:200, mouse monoclonal estrogen receptor 6F11 (Novocastra La., UK) at 1:50, and mouse monoclonal progesterone receptor 1A6 (Novocastra La.) at 1:100. Mouse monoclonal estrogen receptor antibody 6F11 and mouse monoclonal progesterone receptor antibody 1A6 were applied to the slides in a moist chamber at room temperature for 24 hours and 1 hour, respectively. The slides were washed with Tris-buffered saline, and biotinylated link antibody and streptoavidin peroxidase were then applied to each slide for 30 minutes, respectively. At all the experimental steps, the slides were washed twice for 5 minutes. Peroxidase staining was performed with 3, 3' diaminobenzidine tetrahydrochloride (DAKO) for 5 minutes; this was followed by counterstaining with Mayer's hematoxylin, then dehydration and mounting. The positive control for leptin staining was the normal adipose tissue, and that for OBR was the tissue from colon cancer. Immunostaining of >10% of the invasive cancer cells was regarded as positive for leptin and OBR. Samples with scores >2+ were regarded as positive for HER-2/neu. Histologic nuclear grading of the surgical specimens was performed according to the Bloom-Richardson's criteria (11), and the lymphatic invasion was analyzed.
The information on disease stage, menopause, obesity, treatment modalities and disease-free survival rate were obtained from the medical records. Statistical analyses were performed using SPSS software version 11.0 (SPSS Inc., Chicago, IL). The prognosis with respect to the degree of obesity, the expression of leptin and OBR, the state of menopause and treatment with tamoxifen was determined by using the t test, κ2-test, Kaplan-Meier curves and the log rank test. A value of p<0.05 was considered to be statistically significant.
The median age of the 95 patients with early-stage breast can cer was 48 years. The median duration of follow-up was 48.5 months, and 14 (14.7%) patients showed recurrence during thefollow-up period. The patients' BMIs were evaluated at the time of diagnosis. The median body mass index (BMI) was 22.7 kg/m2. When the BMI was ≥25 kg/m2, then this was defined as obesity and a BMI between 23 kg/m2 and 24.9 kg/m2 as overweight; obesity was observed for 42 (44.2%) patients and 20 (21.1%) patients were overweight. Forty-five (47.4%) patients were past menopause (Table 1). Twenty-three (24.2%) patients had stage I breast cancer, 44 patients had stage IIa breast cancer and 28 (29.8%) patients had stage IIb breast cancer. Thirty (31.6%) patients had lymph node metastasis (Table 1).
Sixty-one patients (64.2%) received modified radical mastectomy and thirty-four patients (35.8%) received breast conserving surgery. Thirty-seven (38.9%) patients received radiation therapy. Seventy-five (78.9%) patients received adjuvant chemotherapy after surgery. One patient received neoadjuvant chemotherapy. Eighty-six (90.5%) patients who were positive for estrogen or progesterone receptor received maximum tamoxifen therapy for 5 years and the median duration of therapy was 41 months (range: 12~60 months).
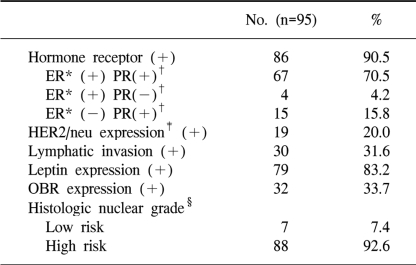
As for the histological nuclear grading of breast cancer, 7 (7.4%) patients were in the low-risk group and 88 (92.6%) in the high-risk group (grades 2 and 3). Nineteen (20.0%) patients showed HER-2/neu positivity (immunoreactivity >2+), 79 (83.2%) patients showed the expression of leptin, and 32 (33.7%) patients showed the expression of OBR (Table 2).
The immunohistochemical findings are shown in Fig. 1: Fig. 1A reveals the antileptin antibody-stained tumors, and Fig. 1B reveals the tumors with negative staining and the adipose tissues with positive staining. The staining results with anti-OBR antibody are shown in Fig. 1C and D: Fig. 1C reveals tumors with positive staining, and Fig. 1D reveals tumors with negative staining. The positive control for leptin staining was the normal adipose tissue, and that for OBR was colon cancer tissue. Immunostaining of >10% of the invasive cancer cells was regarded as positive for leptin and OBR. Overall, the expres-sions of leptin and OBR were 83.2% and 33.7%, respectively (Table 2).
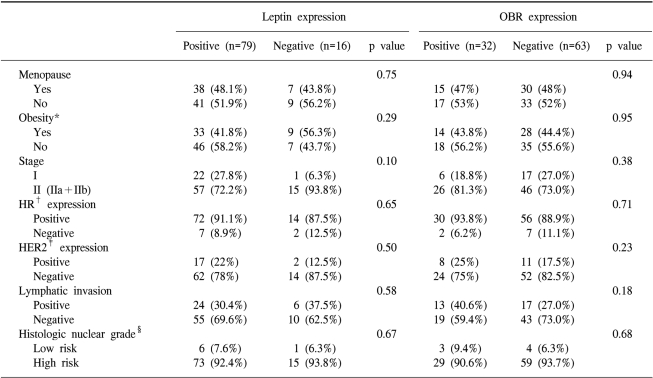
The patients' clinical characteristics, including menopause, obesity, disease stage and lymph node metastasis, were not related to the expression of leptin and OBR (Table 3). The expression of estrogen or progesterone receptor, the expression of HER-2/neu, the presence of lymphatic invasion and the histologic nuclear grading were not related to the expression of leptin and OBR (Table 3). There was no difference for the chemotherapeutic regimen (the anthracycline based regimen vs. the non-anthracycline containing regimen) and the operative method (modified radical mastectomy vs. breast conserving surgery) according to the leptin or OBR expression.
Of the 45 postmenopausal patients, 38 (84%) patients showed a leptin expression and 15 (33%) patients showed an OBR expression. Of the 42 obese patients, 33 (79%) patients showed the expression of leptin and 14 (33%) patients showed the expression of OBR. Of the 86 hormonal receptor (HR)-positive patients, 72 (84%) patients showed a leptin expression and 30 (35%) patients showed the expression of OBR. Of the 21 patients who were HR-positive, postmenopausal and obese, 18 (86%) patients showed a leptin expression and 7 (33%) patients showed an OBR expression. When we performed subgroup analysis, the chemotherapeutic regimen, operation method, disease stage, HER-2/neu expression, lymphatic invasion and histologic nuclear grade were not different according to the expression of leptin or OBR in the postmenopause patients, the HR-positive patients and the obese patients, respectively. These parameters were also not different according to the expression of leptin or OBR in the postmenopausal HR-positive patients, in the postmenopausal obese patients, in the HR-positive obese patients and in the postmenopausal HR-positive obese patients, respectively.
The median follow-up duration was 48.5 months, and the over-all 5-year survival rate was 82%. In terms of the disease free survival rate (DFS), there was not a significantly difference according to the expression of leptin or OBR (Fig. 2). DFS was not significantly different according to the existence of obesity (obese vs. non-obese, p=0.66). When we analyzed DFS for the obese patients, there was no difference in DFS according to the expression of leptin or OBR. When we analyzed DFS for the postmenopause patients, there was no difference in DFS according to the expression of leptin or OBR. When we analyzed DFS for the subgroups of postmenopausal obese patients, there was no difference in DFS according to the expression of leptin or OBR.
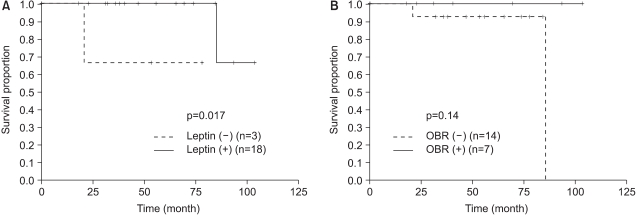
Tamoxifen therapy was not significantly related to DFS, as evaluated by immunohistochemical staining for leptin and OBR (Fig. 3). When we analyzed DFS for the HR-positive group that was treated with tamoxifen, the leptin and OBR expression showed no difference for DFS. When we analyzed DFS for the subgroups of the postmenopausal, HR-positive patients (Fig. 4) and the HR-positive, obese patients who were treated with tamoxifen, the leptin and OBR expressions showed no significant difference in DFS. When we analyzed the postmenopausal, HR-positive, obese patients who were treated with tamoxifen, the DFS of the leptin-positive group was higher than that of the leptin-negative group (p=0.017) but DFS according to the OBR expression was not different (Fig. 5).
It has long been recognized that leptin is an important cytokine for lipid metabolism and energy balance, and both of these are closely related to the development of breast cancer. However, the relationship between the serum leptin levels and the risk for breast cancer is still undetermined. Tessitore et al (12) have reported that serum leptin levels are significantly related to the development of breast cancer, regardless of menopause. In contrast, Mantzoros et al (13) have reported that there is no significant correlation between the serum leptin levels and the development of breast cancer. Ishikawa et al (7) have recently studied the expression of leptin and long-isoform OBR with using immunohistochemistry, and they found that the expression of leptin and OBR was stronger in breast cancer tissue than in normal tissue, and also that distant metastasis occurred more frequently with the increasing expression of leptin and OBR.
In this study, we attempted to observe the relationship between the expression of leptin and long-isoform OBR and the clinicopathological characteristics of early breast cancer patients. The patients' clinical characteristics, including menopause, obesity, the disease stage and lymph node metastasis, were not related to the expression of leptin and OBR (Table 1, 2). The expression of estrogen or progesterone receptor, the expression of HER-2/neu, the presence of lymphatic invasion and the histologic nuclear grade were not related to the expressions of leptin and OBR (Table 3).
We also attempted to observe the relationship between the expressions of leptin and long-isoform OBR and the prognosis of breast cancer in patients with early-stage breast cancer. The DFS for the patients with the expressions of leptin and long-isoform OBR was not significantly different from those patients without the expressions of leptin and long-isoform OBR (Fig. 2).
Garofalo et al (14) found that when faslodex (fulvestrant; Astra Zeneca, UK) was administered to a breast cancer cell line, tumor cell proliferation was inhibited by completely blocking the estrogen receptor (ER), but when it was administered in combination with leptin, the faslodex effects were markedly decreased. The leptin activates ER and then it can activates the signaling pathway for MAPK without estrogen (4). Based on these findings, it is inferred that the local action of leptin is related to tamoxifen resistance.
In this study, all of the patients with hormone receptor were maximally treated with tamoxifen for 5 years and we observed the effect of tamoxifen treatment on DFS for the hormone receptor positive patients according to the leptin and OBR expressions. When we analyzed DFS in the tamoxifen treated postmenopausal positive hormone receptor expression, the leptin and OBR expressions showed no correlation with DFS (Fig. 4). When we analyzed the postmenopause hormone receptor positive obese patients who were treated with tamoxifen, the leptin positive expression group showed statistically significant higher DFS (p=0.017), but the OBR expression showed no significant difference for DFS (Fig. 5). This result was opposite to our expectations. Yet the patients number in our study was too small (n=21) to form a solid conclusion. This study had a limitation that the serum leptin levels were not measured before surgery and during the follow-up period. The changes in local leptin activity according to the serum leptin levels are still unknown. In this study, we evaluated, via immunohistochemistry, the long- isoform OBR, which is known to have the main signal transduction function. Miyoshi et al (15) have recently found that by analyzing the mRNA expression of both long-isoform and short-isoform OBRs in breast cancer tissue, the patients with high plasma leptin levels or high intratumoral leptin mRNA levels and who had the expression of both the long-isoform and short-form of OBR showed a poor prognosis. However, they reported that the plasma leptin levels, the intratumoral leptin mRNA expression and the OBR expression did not correlate with breast cancer as a single prognostic factor, and this suggests that they affect the prognosis of breast cancer in a combined fashion. Considering that the short-isoform OBR is widely distributed and has a high expression rate, further study on the expression of short-isoform OBR is needed to determine the local action of leptin.
The results of this study may suggest that the local expressions of leptin and long-isoform OBR are not related to obesity, the expression of hormone receptors or to menopause in early-stage breast cancer patients. The expressions of leptin and long-isoform OBR in breast cancer tissue may not affect the prognosis of breast cancer. Further research with a larger sample size is needed to elucidate the role of the leptin system with respect to various factors
References
1. Cleary MP, Maihle NJ. The role of body mass index in the relative risk of developing premenopausal versus postmenopausal breast cancer. Proc Soc Exp Biol Med. 1997; 216:28–43. PMID: 9316608.

2. Edman CD, Aiman EJ, Porter JC, MacDonald PC. Identifi cation of the estrogen product of extraglandular aromatization of plasma androstenedione. Am J Obstet Gynecol. 1978; 130:439–447. PMID: 629288.
3. Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, et al. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003; 278:28668–28676. PMID: 12734209.

4. Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996; 15:2174–2183. PMID: 8641283.

5. Shimizu H, Shimomura Y, Nakanishi Y, Futawatari T, Ohtani K, Sato N, et al. Estrogen increases in vivo leptin production in rats and human subjects. J Endocrinol. 1997; 154:285–292. PMID: 9291839.

6. O'Brien SN, Welter BH, Price TM. Presence of leptin in breast cell lines and breast tumors. Biochem Biophys Res Commun. 1999; 259:695–698. PMID: 10364481.
7. Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004; 10:4325–4331. PMID: 15240518.

8. Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006; 12:1447–1453. PMID: 16533767.

9. Savouret JF, Rauch M, Redeuilh G, Sar S, Chauchereau A, Woodruff K, et al. Interplay between estrogens, progestins, retinoic acid and AP-1 on a single regulatory site in the progesterone receptor gene. J Biol Chem. 1994; 269:28955–28962. PMID: 7961858.

10. Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002; 20:3628–3636. PMID: 12202663.

11. Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer. A study of 1049 cases of which 359 have been followed for 15 years. Br J Cancer. 1957; 11:359–377. PMID: 13499785.
12. Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, et al. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000; 5:421–426. PMID: 10719061.

13. Mantzoros CS, Bolhke K, Moschs S, Cramer DW. Leptin in relation to carcinoma in situ of the breast: a study of premenopausal cases and controls. Int J Cancer. 1999; 80:523–526. PMID: 9935151.
14. Garofalo C, Sisci D, Surmacz E. Leptin interferes with the effects of the antiestrogen ici 182,780 in mcf-7 breast cancer cells. Clin Cancer Res. 2004; 10:6466–6475. PMID: 15475434.

15. Miyoshi Y, Funahashi T, Tanaka S, Taguchi T, Tamaki Y, Shimomura L, et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006; 118:1414–1419. PMID: 16206269.

Fig. 1
Immunohistochemical staining of leptin (A, B) and OBR (C, D). (A) Positive staining of tumor tissue (×100). (B) Negatively stained tumor and positively stained adipose tissue (×200). (C) Positively stained tumor and negatively stained adipose tissue (×100). (D) Negatively stained tumor (×40).
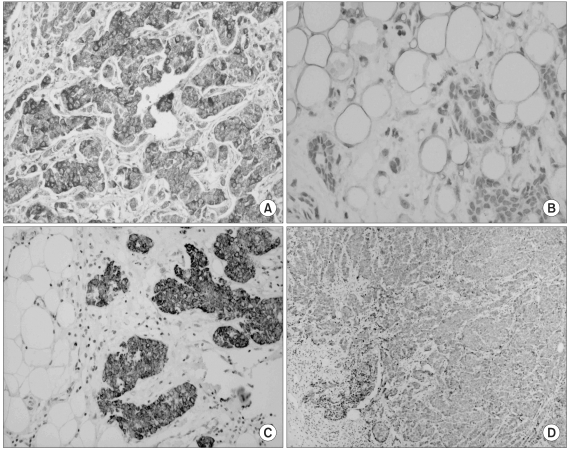
Fig. 2
Disease-free survival for all patients according to the (A) leptin and (B) leptin receptor (OBR) expressions.
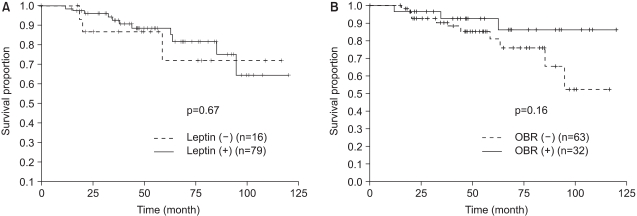
Fig. 3
Comparison of disease-free survival for patients with hormone (estrogen or progesterone) receptor expression between the groups with and without leptin/leptin receptor (OBR) expression.
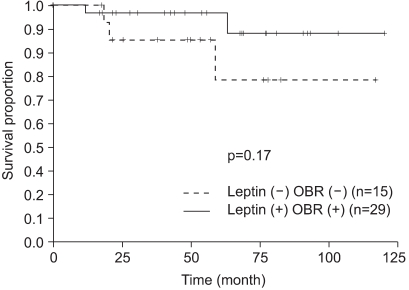
Fig. 4
Disease-free survival for postmenopausal patients with a hormone receptor expression (estrogen receptor or progesterone receptor) and who were treated with tamoxifen according to the (A) leptin expression and (B) the OBR expression.
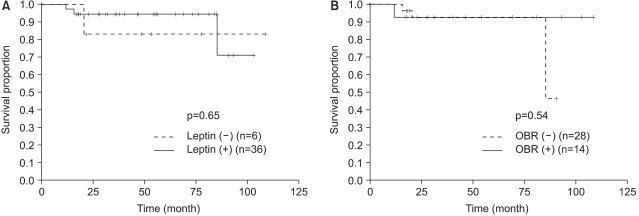
Fig. 5
Disease free survival for obese post-menopause patients with hormone receptor expression (estrogen or progesterone receptor) according to (A) the leptin expression and (B) the OBR expression.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download