Abstract
Purpose
Sodium butyrate (NaBT) is principally a histone deacetylase (HDAC) inhibitor, and it has the potential to arrest HPV-positive carcinoma cells at the G1 to S phase transition of the cell cycle. The aim of study was to determine whether phosphatidylinositol 3-kinase (PI3K) inhibition can enhance the inhibitory effect of NaBT on a human cervical cancer cell line (HeLa).
Materials and Methods
Cervical cancer cells (HeLa) were treated with NaBT alone or in combination with the PI3K inhibitors wortmannin or LY294002. Cell viability analysis and FACS analysis were carried out. The expressions of the cell cycle related proteins were evaluated by Western-blot analysis.
Results
Inhibition of PI3K enhanced NaBT-mediated apoptosis and this decreased the HeLa cell viability. Either wortmannin or LY294002, combined with NaBT, enhanced the activation of caspase 3 and caspase 9, and this enhanced the subsequent cleavage of poly (ADP-ribose) polymerase (PARP). Cervical cancer cells were arrested in the subG1 and G2/M phase, as was detected by FACS analysis. NaBT treatment in combination with PI3K inhibitors showed the increased expression of the CDK inhibitors p21Cip1/Waf1 and p27Kip1, in a p53 dependent manner, and also the increased dephosphorylation of Rb whereas there was a reduction in the expression levels of cyclin A, cyclin D1 and cyclin B1.
Conclusion
The results demonstrate that inhibition of PI3K enhances NaBT-mediated cervical cancer cell apoptosis through the activation of the caspase pathway. Moreover, these findings will support future investigation using the PI3K inhibitors in combination with adjuvant treatment for treating carcinoma of the cervix.
Cervical cancer is the second most common cancer among women according to the most recent estimates of the global cancer incidence, and it remains a significant health care problem throughout the world. Radiation therapy plays a pivotal role in the treatment of advanced-stage cervical cancer. However, roughly 40% to 95% of the women diagnosed with advanced-stage cervical cancer will succumb to their disease (1,2). The intracellular communication pathways leading to promotion of tumor growth, mitotic cell death or apoptosis provide promising targets for intervention in the complex process of radiosensitization.
Among gynecologic cancers, a role for phosphatidylinositol 3-kinase (PI3K) in cervical carcinogenesis has been recently suggested. PI3K is a down-regulator of the Ras signaling pathway and it is a ubiquitous lipid kinase that's involved in receptor signal transduction by the tyrosine kinase receptor. P13K is involved in a number of diverse cellular processes such as growth and transformation, membrane ruffling, vesicular trafficking and cell survival (3~5). PI3K catalyzes the phosphorylation of the inositol ring at the D3 position in a variety of phosphoinositide substrates to form 3-phosphorylated phosphoinositides (6). The mutated ras protoncogene drives cell proliferation and it suppresses the apoptotic pathways through the PI3K pathways. Furthermore, a mutation in the Ras gene and the overexpression of PI3K have been shown to coexist with oncogenic HPV in cervical cancer, and this promotes a faster progression of HPV immortalized epithelial cells to become invasive tumors. All these findings suggest that the persistently high-level expression of the E6 and E7 genes in cervical epithelial cells and the mutual assistance from the cellular factors that exist in many forms are essential for driving complete tumor progression in vivo (7).
Sodium butyrate (NaBT) is a naturally occurring fatty acid that is a sodium salt of n-butyric acid, and it has the capacity to induce apoptosis in transformed cells (8). NaBT is principally a histone deacetylase (HDAC) inhibitor with the potential to arrest HPV-positive carcinoma cells in the G1 to S phase transition of the cell cycle, and this process is paralleled by an up-regulation of the cyclin dependent kinase inhibitors (CKIs) p21Cip1/Waf1 (p21) and p27Kip1 (p27) and a complete loss of cdk2 activity, as well as there is an increase in the concentration of free E2F, which prepares the cell to undergo apoptosis (9). NaBT was shown to be effective for reducing cell proliferation and inducing apoptosis in breast cancer cells, the human colonic tumor cell lines and the HeLa cervical carcinoma cell lines (10~12). However, the clinical trials that have evaluating NaBT as a therapeutic agent for treating malignancies have yielded disappointing results, mostly because of its short bioavailability, which ranges between 5~6 min in the plasma (13). For this reason, we postulate that PI3K inhibition can augment the differentiation of cervical cancer cells that's induced by NaBT.
In this study we set out to analyze the effect of NaBT on the in vitro grown cervical cancer cell line HeLa, in combination with the PI3K inhibitors wortmannin or LY294002. Furthermore, we also determined the cell viability, the expression levels of various cell cycle regulatory proteins, the cell cycle stages and the induction of apoptosis.
The human cervical carcinoma cell line HeLa was obtained from the American Type Culture Collection (Rockville, MD). HeLa cells were cultured in DMEM medium. All the media was supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 g/ml streptomycin. NaBT and wortmannin were purchased from Sigma (St. Louis, MO), and LY294002 was obtained from Calbiochem (San Diego, CA). The antibodies (p27, p21, p53, pRb, beta-tubulin, CDK2, CDK4, cyclin A, cyclin E, cyclun B1 and cyclin D1) used for detection were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The Immobilon P membranes for Western blotting were from Millipore Corp. (Bedford, MA), and the X-ray film was purchased from Eastman Kodak (Rochester, NY). The enhanced chemiluminescence system for Western immunoblot analysis was purchased from Amersham (Arlington Heights, IL).
MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay was done to determine the viability of cells when they were treated with NaBT alone or when they were treated with a combination of NaBT and the PI3K inhibitor wortmannin. The HeLa cells were seeded at a density of 5×103 cells in 0.2 ml of media that was supplemented with 10% FBS in each well of a 96 well plate. NaBT was added at various concentrations one day after inoculation and the cells were incubated for 24 hours. Cellular viability was evaluated based on the reduction of MTT to formazan. After incubation for 4 h with MTT (0.5 mg/ml) at 37℃ and then isopropanol-HCl treatment, the absorbance of the solubilized MTT formazan products was measured at 570 nm. From this, the concentration of NaBT was selected that showed maximum reduction in cell viability. Various concentration of wortmannin was added with the selected concentration of NaBT, and the cell viability was evaluated as described above.
Flow cytometry was performed tg ascertain the effects of NaBT and the combinations of NaBT with wortmannin or LY294002 on the cell cycle distribution of HeLa cells.
1×106 cells/10 ml media were treated with various concentrations of sodium butyrate and a selected concentration of sodium butyrate with various concentrations of wortmannin and LY294002. The treated cells were pelleted by low-speed centrifugation and the pellet was resuspended with PBS. The cells were fixed overnight at 4℃ in ice-cold 70% ethanol. The fixed cells were harvested by centrifugation, resuspended in PBS that contained RNase A (100µg/ml) and propidium iodide (50µg/ml). The ethanol-fixed and propidium iodide-stained cells were analyzed for their DNA content in a FACS sort flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) (BDIS). Data analysis was performed using CellQuest (BDIS) and Modfit software (Verity Software House, Topsham, ME).
Total protein extracts were prepared from the cells treated with sodium butyrate and a combination of sodium butyrate with wortmannin or LY294002. The cells were lysed in lysis buffer that contain 10 mM Tris (pH 7.4), 5 mM EDTA, 130 mM NaCl, 1% Triton X-100, PMSF (10µg/ml), leupeptin (10µg/ml), aprotinin (10µg/ml), 5 mM phenanthroline and 28 mM/L benzamidine-HCl at 4℃ for 30 min. The lysates were clarified by centrifugation (10,000×g for 30 min at 4℃), and the protein concentrations were determined using Bio-Rad Protein Assay Reagent (Bio-Rad, Richmond, CA) and by following the manufacturer's suggested procedure. Briefly, the total protein (100µg) was resolved on an 8~12% polyacrylamide gel and it was transferred to Immobilon-P nylon membranes (Millipore, Bedford, MA). The filters were incubated overnight at 4℃ in a blocking solution (Tris-buffered saline containing 5% nonfat dried milk and 0.1% Tween 20), and this was followed by a 1 h incubation with the primary antibodies. The filters were washed three times in a blocking solution and then they were incubated with horseradish peroxidase-conjugated second antibodies for 1 h. After three additional washes, the immune complexes were visualized by the enhanced chemiluminescence detection system. The antibodies used for detection were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) tubulin was used as a loading control.
The statistical significance of the difference between the inter-group comparisons was obtained by using Student's t-test. The data were expressed as means±SDs, and they were representative of at least three independent experiments. p values <.05 were deemed statistically significant.
HeLa cells were treated for 24 h with various concentrations of sodium butyrate alone and then combinations of NaBT and a PI3K inhibitor. The cell viability was scored by MTT assay and then compared with the control. Cell growth was markedly inhibited by sodium butyrate at a 5 mM/L concentration. This served as the combination concentration of sodium butyrate with PI3K inhibitors (wortmannin or LY294002) in the rest of the experiments. Co-treatment of sodium butyrate and wortmannin was very effective in inhibiting growth, and this was more than using sodium butyrate alone. Wortmannin at a 400 nM/L concentration showed the maximum reduced cellular viability (Fig. 1).
Perturbations in the cell-cycle progression of HeLa cells were associated with exposure to sodium butyrate, wortmannin andLY294002. The cell-cycle distribution of the HeLa cells treated with sodium butyrate and PI3K inhibitors was assessed by flow cytometry. The histogram of the HeLa cells treated with sodium butyrate and sodium butyrate with wortmannin or LY294002 revealed increased an percentage of cells in the sub G1 and G2/M phase. At a 5 mM/L concentration, sodium butyrate induced the maximum sub G1 arrest. Combinations of sodium butyrate with wortmannin or LY294002 also showed sub G1 arrest and as predicted, the combinational treatment was more effective than the individual effect of the respective compounds (Fig. 2).
Western blot analysis revealed the expression of various cell cycle and apoptosis-related proteins. The increased expressions of p21 and p53 and the dephosphrylation of pRb were detected in the HeLa cells treated with 5 mM/L concentrations of sodium butyrate alone. HeLa cells treated with sodium butyrate and PI3K inhibitors revealed the increased expressions of p21, p27 and p53 compared to treatment with sodium butyrate alone. However, co-treatment of sodium butyrate with LY294002 showed a less increased expression of p21, p27 and p53 compared to treatment with sodium butyrate alone. Dephosphorylation of pRb was also noted during the treatment with sodium butyrate and PI3K inhibitors (Fig. 3). The expression levels of these proteins were well correlated with the increase in the sub G1 fraction. The amount of cdk2 was not affected by sodium butyrate alone or with co-treatment with PI3K inhibitors. The reduced expressions of cyclin A, cyclin B1, cyclin D1 and cdk4 were detected with the treatment of sodium butyrate alone and or with co-treatment with PI3K inhibitor. However, the increased expression of cyclin E was detected (Fig. 4).
In order to determine the molecular mechanisms of cell death that's induced by these agents on HeLa cells, the activation of caspases and the PARP cleavage were evaluated. As shown in Fig. 5, the combination of sodium butyrate and PI3K inhibitors resulted in enhanced activation of caspase 9 and 3. In addition, the cleavage of PARP was noted (Fig. 5). Taken together, these results demonstrate that the initiator caspase 9 and the effector caspase 3 are involved in mediating the augmented induction of apoptosis in the HeLa cells co-treated with sodium butyrate and the PI3K inhibitors.
Although HPV infection is the main factor associated with cervical carcinoma, additional factors are needed for the development of cervical cancer (14). Increased PI3k activity plays an important role in the increased proliferation and survival of various cancers, including cervical cancer. To unravel the molecular mechanism of sodium butyrate inhibition on human papillomavirus (HPV)-transformed cells, we used a HPV 18-positive cervical carcinoma cell line (HeLa).
In this study we demonstrated that inhibition of the PI3K/Akt pathway enhances sodium butyrate-induced apoptosis in the cervical carcinoma cell line (HeLa) in vitro. Either wortmannin or LY294002 in combination with NaBT increased the activation of caspase-9 and caspase-3 and the subsequent cleavage of PARP in the HeLa cells. These findings suggest that those agents targeting the PI3k pathway may represent a novel adjuvant therapy for the treatment of cervical cancers. The increased activity of both PI3k and the downstream protein Akt/PKB suggests a role for this pathway in enhanced cancer cell survival (15~17). In fact, PI3k activity was increased both in cervical cancer and in the cervical cancer cell lines due to the amplification of chromosome arm 3q, which is the most consistent aberration seen in cervical cancer (18). In this study, we did not identify an obvious inhibitory effect for either wortmannin or LY294002, when they were used as single agents, on the in vitro growth of the HeLa cells. In contrast to treatment with the PI3k inhibitors alone, the combination of PI3k inhibitors with the short chain fatty acid sodium butyrate enhanced apoptosis, as was demonstrated by the increased number of cells in the sub G1 phase of the cell cycle and also by the reduced cell viability. In addition, the combination of wortmannin and sodium butyrate increased the activities of caspase-9 and caspase-3 and also the rate of PARP cleavage. In the present study, we show that sodium butyrate can efficiently abrogate G1 to S transition. As a consequence, programmed cell death is induced, which we confirmed by the increased number of cells in the sub G1 phase of the cell cycle. In detail, by monitoring the cell cycle regulatory proteins, it became evident that sodium butyrate can modulate the expression of both cyclins and cyclin-dependent kinase (cdk) inhibitors more effectively when it is used in combination with PI3k inhibitors. We have shown here that sodium butyrate in combination with PI3K inhibitors can circumvent the HPV oncogene function via the up-regulation of the CDK inhibitors p21 and p27 in a p53 dependent manner, and also via the dephosphorylation of Rb and the reduction in the expression levels of cyclin A, cyclin D1 and cyclin B1. The increase in CDK inhibitors p21 and p27 did not seem to affect the expression levels of cdk2 and cdk4.
These findings suggest that the combination treatment is more effective than treatment with either agent alone. Sodium butyrate treatment can induce differentiation and cell death through a number of mechanisms, including inhibition of HDAs (9,11), and the HDAC inhibitors have received much attention during the last years due to their capability to induce cell differentiation, growth arrest and apoptosis (19). Moreover, sodium butyrate has been shown to stimulate Akt activity; therefore, the enhanced effect of the PI3k inhibitors may come about through the inhibition of sodium butyrate-mediated Akt activity. Collectively, these findings suggest that the selective inhibition of PI3k may be useful in combination with chemotherapeutic agents to target other cellular pathways. Both Wortmannin and LY294002 have been shown to inhibit other enzymes in addition to PI3k, and such inhibition usually occurs at much higher concentrations than those used in our experiments (20,21). Wortmannin at concentrations that ranged from 20 nM to 2µM appeared to be specific for PI3k and it failed to inhibit PI4-kinase, protein kinase A, protein kinase C and protein kinase G (22). However, it is possible that wortmannin, at the concentrations used in this study (400 nM), may inhibit DNA-dependent protein kinase, a member of the PI3k family (23), and so contribute to enhanced NaBT-induced apoptosis. Future studies will better delineate the potential role of these kinases in the inhibitory effect exerted by PI3k inhibitors.
In summary, we have shown that PI3k inhibition enhances the apoptosis induced by sodium butyrate in the cervical cancer cell line HeLa by inducing a block at the G1 to S transition, and the subsequent apoptosis may have important implications for the treatment of cervical cancer.
Notes
This study was supported by grant # RTI 04-03-02 from the Regional Technology Innovation Program of the Ministry of Commerce, Industry and Energy (MOCIE), and it was partly supported by Grant # R13-2002-028-01001-0 from the Basic Research Program of the Korea Science and Engineering Foundation (KOSEF) for Chronic Disease Research (CDR) Center at Keimyung University.
References
1. Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003; 159:283–300. PMID: 12600231.
2. Ma BB, Bristow RG, Kim J, Siu LL. Combine-modality treatment of solid tumors using radiotherapy and molecular targeted agents. J Clin Oncol. 2003; 21:2760–2776. PMID: 12860956.
3. Roche S, Koegl M, Courtneidge SA. The phosphatidylinositol 3-kinase α is required for DNA synthesis induced by some, but not all, growth factors. Proc Natl Acad Sci USA. 1994; 91:9185–9189. PMID: 8090789.
4. Philpott KL, McCarthy MJ, Klippel A, Rubin LL. Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol. 1997; 139:809–815. PMID: 9348296.

5. Davidson HW. Wortmannin causes mistargeting of procathepsin D. Evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J Cell Biol. 1995; 130:797–805. PMID: 7642698.

6. Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999; 11:219–225. PMID: 10209156.

7. Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci USA. 1996; 93:2930–2935. PMID: 8610145.

8. Cummings JH. Short-chain fatty acids in the human colon. Gut. 1981; 22:763–779. PMID: 7028579.
9. Finzer P, Kuntzen C, Soto U, zur Hausen H, Rosl F. Inhibitors of histone deacetylase arrest cell cycle and induce apoptosis in cervical carcinoma cells circumventing human papillomavirus oncogene expression. Oncogene. 2001; 20:4768–4776. PMID: 11521189.

10. Finzer P, Ventz R, Kuntzen C, Seibert N, Soto U, Rosl F. Growth arrest of HPV-positive cells after histone deacetylase inhibition is dependent of E6/E7 oncogene expression. Virology. 2002; 304:265–273. PMID: 12504567.
11. Mandal M, Kumar R. Bcl-2 expression regulates sodium butyrate-induced apoptosis in human MCF-7 breast cancer cells. Cell Growth Differ. 1996; 7:311–318. PMID: 8838861.
12. Coradini D, Pellizzaro C, Marimpietri D, Abolafio G, Daidone MG. Sodium butyrate modulates cell cycle-related proteins in HT29 human colonic adenocarcinoma cells. Cell Prolif. 2000; 33:139–146. PMID: 10959623.

13. Newmark HL, Young CW. Butyrate and phenylacetate as differentiating agents: practical problems and opportunities. J Cell Biochem Suppl. 1995; 22(suppl):247–253. PMID: 8538206.

14. Alonio LV, Picconi MA, Dalbert D, Mural J, Bartt O, Bazan G, et al. Ha-ras oncogene mutation associated to progression of papillomavirus induced lesions of uterine cervix. J Clin Virol. 2003; 27:263–269. PMID: 12878090.

15. Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001; 70:535–602. PMID: 11395417.

16. Benistant C, Chapuis H, Roche S. A specific function for phosphatidylinositol 3-kinase α(p85α-p110α) in cell survival and for phosphatidylinositol 3-kinase β(p85α-p110β) in de novo DNA synthesis of human colon carcinoma cells. Oncogene. 2000; 19:5083–5090. PMID: 11042696.
17. Kermorgant S, Aparicio T, Dessirier V, Lewin MJ, Lehy T. Hepatocyte growth factor induces colonic cancer cell invasiveness via enhanced motility and protease overproduction. Evidence for PI3 kinase and PKC involvement. Carcinogenesis. 2001; 22:1035–1042. PMID: 11408346.

18. Ma YY, Wei SJ, Lin YC, Lung JC, Chang TC, Whang-Peng J, et al. PIK3CA as an oncogene in cervical cancer. Oncogene. 2000; 25:2739–2744. PMID: 10851074.

19. Janson W, Brandner G, Siegel J. Butyrate modulates DNA-damage-induced p53 response by induction of p53-independent differentation and apoptosis. Oncogene. 1997; 15:1395–1406. PMID: 9333015.
20. Yih LH, Lee TC. Arsenite induces p53 accumulation through an ATM-dependent pathway in human fibroblasts. Cancer Res. 2000; 60:6346–6352. PMID: 11103796.
21. Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, et al. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Bio. 1996; 16:1722–1733. PMID: 8657148.

22. Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, et al. The PI 3-kinase/Akt signaling pathway delivers an antiapoptotic signal. Genes Dev. 1997; 11:701–713. PMID: 9087425.

23. Boulton S, Kyle S, Yalcintepe L, Durkacz BW. Wortmannin is a potent inhibitor of DNA double strand break but not single strand break repair in Chinese hamster ovary cells. Carcinogenesis. 1996; 17:2285–2290. PMID: 8968039.

Fig. 1
Growth inhibition in the HeLa cells treated for 24 h with a combined concentration of NaBT with the PI3K inhibitor (wortmannin). Cell viability was measured using the Cell Titer Cell Proliferation Assay and the results are expressed as a % of the control culture conditions. *p<0.05, †p<0.01.
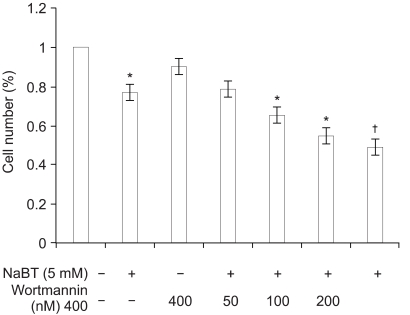
Fig. 2
Effect of a treatment with NaBT alone or NaBT with wortmannin or LY294002 treatment on the cell cycle profile. After treatment for 24 h with 5 mM/L NaBT alone or DMSO only or 5 mM/L NaBT with 400 nM/L wortmannin or 20µM/L LY294002, the HeLa cells were collected, fixed, stained with propidium iodide and analyzed by flow cytometry. The values represent the number of cells in a phase of the cell cycle as a percentage of the total cells.

Fig. 3
Effects of PI3K inhibitors and NaBT on the cell cycle-related genes. HeLa cells were treated for 24 h with 5 mM/L NaBT alone or DMSO only or with 5 mM/L NaBT with 400 nM/L wortmannin or 20µM/L LY294002. The whole cell lysates were prepared and they were used for the detection of each protein expression using antibodies against p21Cip1/Waf1, p27Kip1, pRb and p53 by Western blot analysis. β-tubulin was used as an internal control.
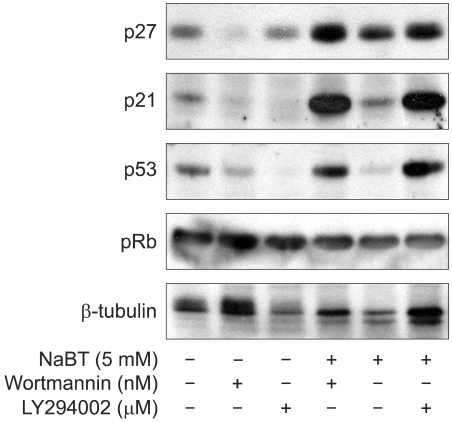
Fig. 4
Effects of PI3K inhibitors and NaBT on cyclins and cdks. The whole cell lysates were prepared and used for the detection of each protein expression with using antibodies against CDK2, CDK4, cyclin A, cyclin B1, cyclin D1 and cyclin E by Western blot analysis. β-tubulin was used as an internal control.
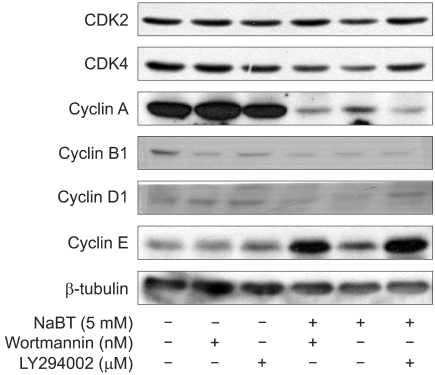
Fig. 5
Effects of PI3K inhibitors and NaBT on caspase activation and PARP cleavage. The whole cell lysates were prepared and used for the detection of each protein expression with using antibodies against caspase 3, caspase 9 and PARP by Western blot analysis. β-tubulin was used as an internal control.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download