Abstract
Purpose
It is well known that human papillomavirus (HPV) is the main cause of cervical neoplasia, and hydrogen peroxide-producing lactobacilli are the most important microorganisms for maintaining the balance of the vaginal ecosystem. The purpose of our study was to investigate the relationship of hydrogen peroxide-producing lactobacilli, cervical neoplasia and high-risk HPV.
Materials and Methods
We enrolled 1138 women with abnormal cervical smears or cervicograms who were referred to the department of Obstetrics and Gynecology at Chonnam National University Medical School. In all of them, 1,138 vaginal swabs were collected for the qualitative assay of hydrogen peroxide producing lactobacilli and 150 cervical swabs were used for the HPV hybrid capture II test without regard to the subjects' pregnancy status. In the non-pregnant women, 880 cervical biopsies and/or loop electrosurgical excision procedures were performed for making the histological diagnosis.
Infection with some genotypes of HPV is the most important risk factor associated with cervical neoplasia, and H2O2 producing lactobacilli are the most important microorganisms that maintain the balance of the vaginal ecosystem. Many authors have suggested that H2O2 producing lactobacilli have antibacterial, antiviral and potential antitumoral effects (1~3).
Therefore, it is hypothesized that H2O2 producing lactobacilli might have some responsibility for the pathogenesis of cervical neoplasia by HPV, and the bacterial distribution might be different for women with cervical neoplasia and/or who are infected high-risk HPV. The purpose of our study was to investigate the relationship of H2O2 producing lactobacilli, cervical neoplasia and high-risk HPV.
We enrolled 1,138 women who had abnormal cervical smears or cervicograms and who were referred to the department of Obstetrics and Gynecology at Chonnam National University Medical School. 1,138 vaginal swabs were collected from all of them for the qualitative assay of H2O2 producing lactobacilli, and 150 cervical swabs for the HPV hybrid capture II (HC-II; Digene, Sliver, Spring, MD) test were also obtained without regard to whether the women were pregnancy. In non-pregnant women, 880 cervical biopsies and/or loop electrosurgical excision procedures (LEEP) were performed for making the histological diagnosis. The results of each test were analyzed by Chi-square test and p values <0.01 were considered statistically significant.
Vaginal swabs were used to obtain samplings from the lateral vaginal wall and they were immediately taken to the laboratory room. The vaginal swabs were removed from the transport medium and then used to inoculate the Mann Rogasa Sharpe media (MRS; Difico, Detroit, MI). The plates were incubated at 37℃ in 7% CO2 for 48 hours and then they were tested for the production of H2O2 via a qualitative assay on a tetrame-thylbenzine (TMB) agar plate. The lactobacilli were presumptively identified by their ability to grow well on MRS. After 48 hours of incubation in an anaerobic glove box at 37℃, the agar plates were exposed the ambient air. The H2O2 that was formed then reacted with the horseradish peroxidase in the agar to oxidize the TMB, causing the colonies of hydrogen peroxide producing lactobacilli to turn blue and so these samples were classified as positive (Fig. 1) (4).
The sample was collected by placing a cytobrush into the exocervix and rotating the brush three times; this was kept frozen at -20℃ in a collection tube (Digene) until needed. The samples of the mixed high risk HPV denatured single-strand DNA were hybridized with RNA probe cocktail. This reaction mixture was placed in a microtiter well that was coated with antibodies for the RNA/DNA hybrid. After this RNA/DNA hybrid-antibody bonding, the mixture was reacted with alkaline phosphatase-conjugated antibodies and then washed, and lumi-Phospho 530 was added to react with the dioxetane-based chemiluminuscent substrate. Alkaline phosphatase was then added to produce luminescent light that was measured with a luminometer (DML 2000™, Digene); the value of the light was expressed in relative light units. A solution containing 1 pg/ml of the HPV 16 DNA was used as the positive control group for the high-risk HPV group. The relative light units for all the samples were set to the degree of relative brightness as compared to the positive control group. This ratio was considered positive when it was ≥1.0 and negative when it was <1.0. The presence of the 13 types of high-risk HPV groups (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) was confirmed in all the samples.
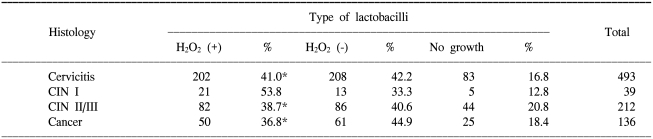
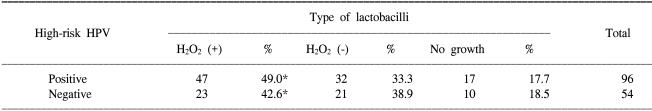
In all the 880 non-pregnant women, both a qualitative assay of H2O2 producing lactobacilli and the cervical histology were performed. As shown in Table 1, there was no significant difference between the distribution of H2O2 producing lactobacilli and the cervical histology. In 150 women without regard to their pregnancy status, both the qualitative assay of H2O2 producing lactobacilli and the HC-II test were performed. As shown in Table 2, there was no significant difference between the distribution of H2O2 producing lactobacilli and the positivity for high-risk HPV.
In the normal vaginal microbial flora found in women of reproductive age, lactobacilli have been shown to be the predominant bacteria; these lactobacteria are gram positive, non-motile rod-like bacteria that are characterized by the ability to grow in acid media, tolerate acid conditions and ferment carbohydrates to produce lactic acid (5~7). More recently, molecular methods have demonstrated Lactobacilli crispatus and L jensenii to be the most common isolates (8).
Lactobacilli, principally the strains that produce H2O2, may have a protective effect against vaginal colonization by pathogenic species such as those that cause bacterial vaginosis (BV), gonorrhea, staphylococci, E. coli, shigella, listeria and possibly human immunodeficiency virus (1,9). This has been explained by many mechanisms. In addition to competitive inhibition of the epithelial and mucosal adherence of pathogens and the inhibition of epithelial invasion by pathogens, lactobacilli also show antimicrobical activity by producing H2O2, acids, bacteriocin and biosulfactants, and they also stimulate mucosal immunity (10). Lactobacilli strains that produce H2O2 have been isolated from 79% to 96% of women who have a healthy vaginal ecosystem. H2O2 has been shown elicit cellular injury by initiating lipid peroxidation, protein oxidation and DNA damage. Klebanoff et al demonstrated that H2O2 producing lactobacilli are present in the vagina of most normal healthy women, but they are absent from most women with BV. In addition, the toxicity of H2O2 producing lactobacilli to Gardnella vaginalis was influenced by several interactive factors that consisted of reactive oxygen species (ROS) (11) Other in vitro studies have also demonstrated the involvement of the peroxidase system in the inhibition of Neisseria gonorrheae in an acidic environment; this inhibition is the result of complex effects from H2O2, acid production and bacteriocin-like compounds. Further, there is a viricidal effect on HIV-1 that affects the heterosexual transmission of the virus (1,12).
By producing metabolites such as acetic acid and lactic acid, a large number of lactobacilli serve to lower the vaginal pH and inhibit the growth of pathogens. Alakomi et al observed that the lactic acid produced by lactobacilli acts as a permeablizer of the gram-negative bacterial outer membrane, allowing other antimicrobial substances produced by the host to penetrate and increase the susceptibility of pathogens to antimicrobial molecules (13).
Lactobacilli have also been shown to activate cells to get them to secrete both inflammatory and anti-inflammatory cytokines. Klebanoff et al demonstrated that H2O2 producing lactobacilli increase TNF-α and IL-1α production, activate NF-κB in THP-1 cells and increase TNF-α production by human monocytes. This suggests the presence in the vaginal fluid of biochemical factors derived from indigenous bacteria that can influence the physiology of the vagina and the host defences (14).
HPV has been identified as a necessary causal agent for cervical squamous neoplasia and it has been linked to the development of neoplasia in several other mucosal sites. The viral oncogenes E6 and E7 are the major players in the viral scheme to evade the immune system and use the host cell replication machinery to survive. Although infection with an oncogenic HPV has been shown to be a necessary cause of cervical neoplasia, it is not a sufficient cause and most of the women infected with a specific HPV will not show evidence the same type 6~12 months later (15,16). Although there is a high incidence and prevalence of HPV in the general population, the majority of infected women appear to clear the virus via an effective immune response (17).
In consideration of the above mentioned studies, we hypothesized that H2O2 producing lactobacilli as a host immune factor might be responsible for the pathogenesis of cervical neoplasia according to high-risk HPV infection and its distribution might be different in the women with cervical neoplasia and/or who are infected high-risk HPV.
Our study unexpectedly showed that there was no significant difference not only between the distribution of H2O2 producing lactobacilli and cervical histology, but also between the distribution of H2O2 producing lactobacilli and the positivity of high-risk HPV. Our results may be explained by the possible protective mechanisms of HPV against H2O2 injury and also that the pathogenesis of cervical neoplasia might be not influenced by the existence of H2O2 producing lactobacilli. This reasoning is indirectly supported by several studies. According to Lee et al's in vitro study, when E6 and E7 proteins were over-expressed in primary astrocytes by using retroviral vectors and the HPV-16 E6 and E7 genes, the E6 and E7 gene products were able to protect cells from H2O2 injury (18~20).
Although there are few reports concerned with H2O2 producing lactobacilli, HPV and cervical neoplasia, lactobacilli as probiotic agents have been investigated for their link with recurrent BV, inflammatory bowel disease, cystic fibrosis, dental caries and irritable bowel syndrome (21,22). The administration of lactobacilli by mouth and tranvaginally has been shown to be safe and to reduce the risk of urinary tract infection, BV and yeast vaginitis. Lactic acid bacteria have been shown to prevent somatic mutations in colon cancer and so they have potential as chemoprotective agents that may affect carcinogen-activating enzymes and carcinogen-deactivating enzymes (23).
Our study had several limitations about sampling, i.e., that only one specimen per every woman was obtained without concerning about menstrual variation of lactobacilli, menopause, the medical history, sexual activity, a normal control group and a long natural history of cervical neoplasia.
This study was a preliminary study to define possible roles of H2O2 producing lactobacilli in the pathogenesis of cervical neoplasia. Irrespective of our results, the idea of using normal flora with antimicrobial activity to prevent and potentially treat cancer is very attractive. Further studies are needed to verify the relationship between H2O2 producing lactobacilli, high-risk HPV and cervical neoplasia.
References
1. Klebanoff SJ, Coombs RW. Viricidal effect of lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J Exp Med. 1991; 174:289–292. PMID: 1647436.

2. Clark RA, Klebanoff SJ. Role of the myeloperoxidase-H2O2-halide system in concanavalin A-induced tumor cell killing by human neutrophils. J Immunol. 1979; 122:2605–2610. PMID: 221588.
3. Clark RA, Olsson I, Klebanoff SJ. Cytotoxicity for tumor cells of cationic proteins from human neutrophil granules. J Cell Biol. 1976; 70:719–723. PMID: 182702.

4. Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, Critchlow CM, et al. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989; 27:251–256. PMID: 2915019.

5. Redondo-Lopez V, Cook RL, Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990; 12:856–872. PMID: 2237129.

6. Wilks M, Wiggins R, Whiley A, Hennessy E, Warwick S, Porter H, et al. Identification and H2O2 production of vaginal lactobacilli from pregnant women at high risk of preterm birth and relation with outcome. J Clin Microbiol. 2004; 42:713–717. PMID: 14766841.
7. Giorgi A, Torriani S, Dellaglio F, Bo G, Stola E, Bernuzzi L. Identification of vaginal lactobacilli from asymptomatic women. Microbiologica. 1987; 10:377–384. PMID: 3695985.
8. Reid G, McGroarty JA, Tomeczek L, Bruce AW. Identification and plasmid profiles of Lactobacillus species from the vagina of 100 healthy women. FEMS Immunol Med Microbiol. 1996; 15:23–26. PMID: 8871112.
9. Dembele T, Obdrzalek V, Votava M. Inhibition of bacterial pathogens by lactobacilli. Zentralbl Bakteriol. 1998; 288:395–401. PMID: 9861683.
10. Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004; 28:405–440. PMID: 15374659.

11. Klebanoff SJ, Hillier SL, Eschenbach DA, Waltersdorph AM. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J Infect Dis. 1991; 164:94–100. PMID: 1647428.
12. Zheng HY, Alcorn TM, Cohen MS. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J Infect Dis. 1994; 170:1209–1215. PMID: 7963715.
13. Alakomi HL, Skytta E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol. 2000; 66:2001–2005. PMID: 10788373.

14. Klebanoff SJ, Watts DH, Mehlin C, Headley CM. Lactobacilli and vaginal host defense: activation of the human immunodeficiency virus type 1 long terminal repeat, cytokine production, and NF-kappB. J Infect Dis. 1999; 179:653–660. PMID: 9952372.
15. Nobbenhuis MA, Helmerhorst TJ, van den Brule AJ, Rozendaal L, Voorhorst FJ, Bezemer PD, et al. Cytological regression and clearance of high-risk human papillomavirus in women with an abnormal cervical smear. Lancet. 2001; 358:1782–1783. PMID: 11734239.

16. Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, Desy M, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999; 180:1415–1423. PMID: 10515798.

17. Steenbergen RD, de Wilde J, Wilting SM, Brink AA, Snijders PJ, Meijer CJ. HPV-mediated transformation of the anogenital tract. J Clin Virol. 2005; 32(Suppl 1):S25–S33. PMID: 15753009.

18. Lee JE, Kim CY, Giaccia AJ, Gifffard RG. The E6 and E7 genes of human papilloma virus-type 16 protect primary astrocyte cultures from injury. Brain Res. 1998; 795:10–16. PMID: 9622584.

19. Lee WT, Lee JE, Lee SH, Jang HS, Giffard RG, Park KA. Human papilloma virus type 16 E7 genes protect astrocytes against apoptotic and necrotic death induced by hydrogen peroxide. Yonsei Med J. 2001; 42:471–479. PMID: 11675674.

20. Chen QM, Merrett JB, Dilley T, Purdom S. Down regulation of p53 with HPV E6 delays and modifies cell death in oxidant response of human diploid fibroblasts: an apoptosis-like cell death associated with mitosis. Oncogene. 2002; 21:5313–5324. PMID: 12149652.

21. Gorbach SL. Probiotics in the third millennium. Dig Liver Dis. 2002; 34(Suppl 2):S2–S7. PMID: 12408431.

22. Cardone A, Zarcone R, Borrelli A, Di Cunzolo A, Russo A, Tartaglia E. Utilization of hydrogen peroxide in the treatment of recurrent bacterial vaginosis. Minerva Ginecol. 2003; 55:483–492. PMID: 14676737.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download