Abstract
Gastric cancer remains a significant problem in terms of global health, and is the most common cancer in Korea. Surgery is the only potentially curative treatment for localized gastric cancer, but most cases present at an advanced stage. Randomized trials have demonstrated that chemotherapy for advanced gastric cancer improves the quality of life and extends survival, by 4~6 months, compared with best supportive care alone. Single agents with a proven activity in a first-line setting include 5-fluorouracil (5-FU), doxorubicin, mitomycin C, cisplatin, taxanes (docetaxel and paclitaxel) and oral fluoropyrimidines (capecitabine and TS-1). Based on the results from several large scale randomized trials, FP (5-FU/cisplatin) and ECF (epirubicin/cisplatin/5-FU) combinations are the most widely used regimen against advanced gastric cancer. Phase II studies of the FP and ECF combination reported a 40~51% response rate in previously untreated patients, and this regimen also produced a significantly higherresponse rate than the FAM (5-FU/doxorubicin/ mitomycin) and FAMTX (5-FU/doxorubicin/methotrexate) regimens, respectively. However, significant treatment related-toxicities and discomfort were reported from ECF, which prevents this combination from becoming the standard treatment regimen. While no one combination chemotherapy regimen is accepted as the standard for advanced gastric cancer, FP is currently considered a suitable reference regimen worldwide. New agents, such as taxane, irinotecan and oxaliplatin, combined with old agents, such as cisplatin and 5-FU, are currently under evaluation to further improve treatment outcomes. Also, oral 5-FU prodrugs are replacing the cumbersome 5-FU long-term infusion due to its convenience and superior toxicity profile. However, the low complete response rate and short response duration are still the main obstacles in the chemotherapy for gastric cancer. Only large scale comparative clinical trials will give clues to improve the results of gastric cancer treatments.
Gastric cancer is one of the most common cancers in the world, which ranks first in frequency among Koreans (1). Curative surgery is the treatment of choice, with recent improvements in the overall survival rate. However, the mortality of patients diagnosed with gastric cancer still remains high, due to many patients being diagnosed in the advanced stages of the disease. More than two-thirds of patients with gastric cancer will have an unresectable disease (2). Although various chemotherapeutic agents, either alone or in combination, have been studies since 1970, the median survival of patients with a metastatic disease remains between 6 and 9 months. Therefore, there is a need for more effective systemic therapy to improve the management of patients with advanced gastric cancer.
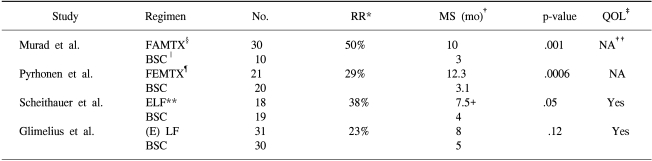
The efficacy of chemotherapy with palliative intent, compared to that of supportive care alone, is now widely accepted. Studies have shown the benefit of combination regimens, such as FAMTX (5-FU, doxorubicin and high-dose methotrexate) or ELF (etoposide, leucovorin and 5-FU) over that of the best supportive care (3~5) (Table 1). The survival advantage was paralleled by an improvement in the quality of life, and the treatment appeared to be cost-effective. However, the survival advantage is small, and no internationally accepted standard regimen has emerged (6). While no one combination chemotherapy regimen is accepted as the standard for advanced gastric cancer, the continuous infusion of 5-FU with cisplatin is currently considered a suitable reference regimen worldwide.
Recently, several new agents have emerged as potential new options for this disease. Promising data have been reported with docetaxel, paclitaxel, irinotecan, oxaliplatin, capecitabine and TS-1. In this article, the results of various clinical trials available in the current literature, as a single agent chemotherapy or combination chemotherapy in patients with advanced gastric cancer, will be discussed.
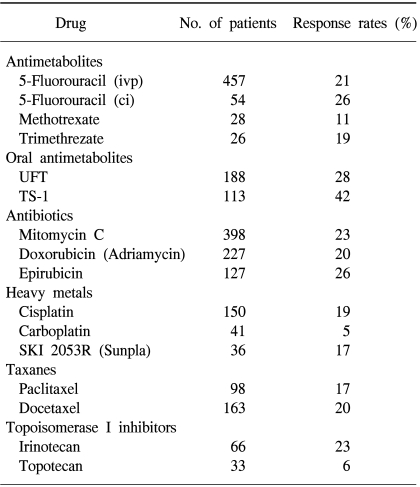
Many of the trials evaluating single agents have been small and uncontrolled, making it difficult to draw firm conclusions regarding their efficacy. The most extensively studied agents are 5-fluorouracil (5-FU), doxorubicin, mitomycin C and cisplatin, with newer cytotoxic agents being the taxanes (docetaxel and paclitaxel), oral fluoropyrimidines (capecitabine and TS-1), oxaliplatin and irinotecan (Table 2).
5-FU is one of the most effective and widely used single agent in patients with advanced gastric cancer (7), and forms part of all the current reference regimens. 5-FU monotherapy, a standard treatment in Japan, is associated with a response rate of approximately 20% and an overall survival time of between 5 and 7 months in phase III randomized studies (8, 9). The modulation of 5-FU with leucovorin has generally enhanced the antitumor efficacy (10,11), and has been shown to have activity in patients who had previously progressed on 5-FU-containing combinations (12).
Mitomycin is also an active single agent in the treatment of gastric cancer. A response rate of around 30% has been reported (13,14), but the clinical use was limited due to delayed myelotoxicity and the occurrence of hemolytic uremic syndrome.
Taxanes (paclitaxel and docetaxel) have been tried as single agents in the treatment of advanced gastric cancer. Paclitaxel was well tolerated, with reported overall response rates ranging between 17 and 23% (15~17). The results from several European, US, Japanese and Korean studies have assessed first-line docetaxel monotherapy in advanced gastric cancer, which have indicated overall response rates ranging from 18 to 24% (18~21). Interestingly, the response rate of docetaxel monotherapy was similar between chemotherapy-naive and previously treated patients. The overall response of 129 eligible patients in a late phase II study of docetaxel in advanced or recurrent gastric cancer conducted in Japan was 17.1% (19,20). Of the 96 patients previously treated with chemotherapy, 16.7% responded, compared with 18.2% of the 33 chemotherapy-naive patients.
Irinotecan (CPT-11,7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxy-camptothecin) is a semi-synthetic plant alkaloid obtained from Camptotheca acuminate. After conversion to its active metabolite, SN-38, irinotecan acts by inhibiting the eukaryotic enzyme, DNA-topoisomerase I (22,23). Irinotecan monotherapy is active in patients with gastric cancer, with response rates in phase II trials ranging from 14 to 23% (24~26). A late phase II trial of irinotecan in advanced gastric cancer patients compared two intravenous dosage schedules: 100 mg/m2 once a week, and 150 mg/m2 once every 2 weeks (26). The overall response rate for the 76 eligible patients was 18%. Of the 56 previously treated patients, 16% responded, compared with 25% of the 20 chemotherapy-naive patients.
Due to its convenient route of administration and pharmacodynamic advantage in mimicking protracted 5-FU infusion, oral 5-FU prodrugs have received increased consideration in recent years. UFT, a combination of uracil and ftorafur, has shown an overall response rate of about 28% in various phase II studies (27~29). The oral fluoropyrimidine, capecitabine, was designed to preferentially generate 5-FU in tumor tissue. This tumor selectivity is achieved through exploitation of the significantly higher activity of thymidine phosphorylase in many tumor tissues (30,31). Capecitabine monotherapy has shown an overall response rate of 28%, with good tolerability, in a phase II study of previously untreated patients with advanced gastric cancer (32). In a larger Japanese clinical trial of 60 patients with previously untreated advanced gastric cancer, a 4-weekly intermittent schedule led to a response rate of 26% and a median survival of 8.8 months (33). TS-1 is a new oral dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine, consisting of tegafur, 5-chloro-2,4-dihydroxypyridine and potassium oxonate, at a molar ratio of 1:0.4:1, which has achieved high efficacy, without increasing gastrointestinal toxicity, based on biochemical modulation theory (34). In two late phase II studies for advanced gastric cancer in Japan, the combined response rate of the two studies was 44.6%, with a very low (2.0%) incidence of grade 3 diarrhea (35,36). The phase II study of TS-1 against gastric cancer in Europe, by the EORTC-Early Clinical Study Group, also revealed high efficacy (37).
Although, randomized trials comparing monotherapy with combination regimens have consistently shown increased response rates in favor of the combination regimens, similar survival durations were usually found (38). The response rates of most single agent treatments ranged from 17 to 44.6%. Since monotherapy has an advantage in terms of toxicity compare with combination treatments, they might be tried in a second line setting. Taxanes, especially, have shown similar response rates in both first and second line treatments, which is very unusual in this type of cancer. Whether different schedule of 5-FU administration (bolus intravenous, continuous intravenous, oral, etc.) could overcome previous 5-FU exposure need to be verified by clinical studies in a second line setting.
Many combinations of cytotoxic chemotherapeutic agents have been developed to improve the response rate and duration of survival of advanced gastric cancer patients. In the late 1980s and early 1990s, FAM (5-FU, doxorubicin, mitomycin-C), FP (5-FU, cisplatin), FAMTX (5-FU, doxorubicin, methotrexate), EAP (etoposide, doxorubicin, cisplatin) and ECF (epirubicin, cisplatin, protracted 5-FU infusion) showed high response rates in phase II trials, but lower response rates and an overall survival of less than 1 year in randomized trials (39).
Comparison of FAMTX with FAM in a prospective randomized study revealed a significantly higher overall response rate (41% versus 9%) and median survival (42 versus 29 weeks) for FAMTX, but with similar toxicities (40). In the initial report on 67 patients, EAP was associated with an overall response rate of 64% (41). A follow-up study, however, suggested a much lower response rate of 33% (42), and compared with FAMTX showed a significantly lower response rate (20% versus 33%) and similar survival (6.1 versus 7.3 months) (43). Based on four toxicity related deaths in the EAP arm, the EAP regimen was not recommended for the treatment of gastric cancer after this study (44).
The European Organization for Research and Treatment of Cancer conducted a phase III trial comparing ELF (etoposide, 5-FU, leucovorin), FUP (infusional 5-FU plus cisplatin) and FAMTX (45). All three groups showed similar efficacies, but the FAMTX group had a disappointing response rate (12%), with a median survival of 6.7 months. In a phase III trial comparing PELF (Cisplatin, epirubicin, leucovorin and 5-FU) with FAMTX, PELF was associated with a significantly higher response rate (38% versus 21%) and higher 12-month survival rate (31% versus 22%) (46). Similar combination treatment with PELF, ECF was associated with an overall response rate of 71% and a median survival of 8.2 months in a phase II study (47). A direct comparison of ECF with FAMTX was attempted, with ECF being superior in terms of both the response rate (45% versus 21%) and median survival (8.9 versus 5.7 months) (48) (Table 3). In a phase III study comparing ECF with MCF (mitomycin, cisplatin and 5-FU), both treatments showed similar response rates and survivals. However, a better quality of life was observed with the ECF treatment (49).
The FP combination achieved an overall response rate of 40% and a median survival of 9 months in two phase II studies (50,51). In a study at Seoul National University, FP was compared either with 5-FU alone or with FAM. The objective response rate in the FP arm was superior to those of the other two treatments (51% versus 26% versus 25%), but there was no statistical difference in the survivals (37 versus 31 versus 29 weeks) (52).
There is some justification for considering the ECF regimen as the most active available combination treatment for advanced gastric cancer. However, when interpreting the ECF data it should be noted that a substantial number of patients included in phase II or III ECF studies had locally advanced disease and were; therefore, not stage IV cases using conventional criteria. Also, more than one-third of the patients in those trials had an adenocarcinoma of the esophagus or gastroesophageal junction, which may be, in essence, a different disease from classical gastric cancer. These considerations may help explain why many oncologists consider the FP regimen to have as good a claim as ECF to the role of the standard treatment.
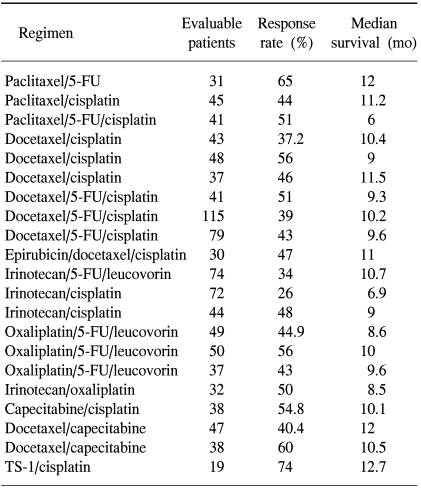
Based on the promising results of taxane monotherapy, taxane-containing combination regimens are actively under evaluation (Table 4). The combination of paclitaxel, cisplatin and 5-FU appears to be a highly active regimen, with acceptable toxicity (53). An overall response rate of 51% was achieved in 41 patients with an advanced gastric carcinoma. The combination of paclitaxel and 5-FU showed a response rate of 65% and a median survival of 12 months in 31 patients with advanced gastric cancer (54). Promising results were also reported with a regimen of paclitaxel and cisplatin (55), with an overall response rate of 44% and a median time to progression and an overall survival of 7 and 11.2 months, respectively.
Several investigator groups have tried the docetaxel with cisplatin combination as treatment for advanced gastric cancer, with overall response rates of 37.2 (56) to 56% (57) and median survivals of 9 (57) to 11.5 months (58) achieved with this regimen. A phase II multicenter trial showed that protracted continuous intravenous 5-FU infusion can be safely added to the docetaxel-cisplatin combination if the docetaxel dose is reduced (59), with an overall response rate and a median survival of 51% and 9.3 months, respectively. To identify which experimental arm should be taken forward into a phase III comparison against cisplatin/5-FU, a multinational effort was mounted to conduct a randomized phase II comparison of docetaxel-cisplatin (DC) versus docetaxel-cisplatin-5-FU (DCF) (60). The response rates in the DC and DCF arms were 32 and 54%, respectively. From an intention-to-treat analysis of the full population, the response rates were 28 and 43% in the DC and DCF arm, respectively. The interim results of a phase III trial comparing DCF to CF (cisplatin plus 5-FU) showed a significantly longer time to progression (5.2 versus 3.7 months) and higher response rate (39% versus 22%) after treatment with the DCF (61). Also, presented data has shown docetaxel to provide a small, but significant, survival benefit when added to CF in advanced gastric cancer. However, poor tolerability and high rate of toxic deaths in this study make the impact of this triplet combination questionable. Instead of the cumbersome long-term 5-FU infusion, oral 5-FU prodrugs, combined with docetaxel/cisplatin, were attempted (62). In all, 52 patients received courses of docetaxel, 60 mg/m2, and cisplatin, 75 mg/m2, administered on day 1. Oral UFT, at 400~600 mg/day, as determined from the body surface area, and leucovorin, at 75 mg/day, were administered for 21 consecutive days from day 1, followed by a 7-day drug-free interval. Four complete responses (7.7%) and 22 partial responses (42.3%) were achieved, giving an overall response rate of 50%. The major toxicity was neutropenia, which reached grade 3/4 in 36 patients (69.3%). The median time to progression, survival duration and response duration were 22 weeks (4 to 156+ weeks), 48 weeks (4 to 156+ weeks) and 24 weeks (6~152 weeks), respectively. Docetaxel, cisplatin, oral UFT and leucovorin combination chemotherapy was effective and tolerable for the treatment of advanced gastric cancer. Epirubicin was added to DC to test the feasibility of the triple combination for the treatment of advanced gastric cancer (63). Although, the response rate was similar to that of other triplet combination chemotherapies for advanced gastric cancer (47%), the median survival duration was 11 months.
Based on promising the activity of irinotecan, a large phase II/III trial (study V306) was undertaken to define its clinical efficacy based on combination therapy in advanced gastric cancer (64). In the initial phase II part of the trial, a total of 146 patients were randomized to receive either irinotecan, 200 mg/m2, plus cisplatin, 60 mg/m2, every 3 weeks, or irinotecan, 80 mg/ m2, weekly plus 5-FU/folinic acid. Neutropenia and its complications were more common when the irinotecan was combined with cisplatin than when it was combined with 5-FU/folinic acid. Diarrhea was more frequent among patients administered irinotecan with 5-FU. The overall response rate of the irinotecan/5-FU/folinic acid (34%) was superior to that of irinotecan/cisplatin (26%). With regard to the time to progression and median survival, the irinotecan/5-FU/folinic acid combination was clearly superior to that of irinotecan/cisplatin (4.5 versus 6.5 months, 6.9 versus 10.7 months). On the basis of these efficacies and safety data, the irinotecan/5-FU/folinic acid combination was adopted for a randomized comparison with 5-FU/cisplatin, which will be reported in the near future (65). However, various schedules of the irinotecan plus cisplatin combination treatments were attempted in advanced gastric cancer patients, with promising results. The overall response rates were between 41.7 (66) and 58% (67), and a median survival of about 9 months. The schedule of irinotecan, 70 mg/m2, on days 1 and 15 and cisplatin, 80 mg/m2, on day 1, every 4 weeks, seemed better in terms of toxicity. A highly tolerable alternative to this regimen is the combination of irinotecan, 60 mg/m2, with low-dose cisplatin, 6 mg/m2 (68). This regimen resulted in a response rate of 52%, with a positive impact on the quality of life in 21 patients who failed previous 5-FU chemotherapy.
Oxaliplatin is a third-generation platinum compound, which has a wide range of antitumor activities. Compared with cisplatin, oxaliplatin appears to have a better safety profile, with minimal cross-resistance to cisplatin (69). Weekly and biweekly 5-FU/folinic acid/oxaliplatin regimens have mainly been explored in colorectal cancer, with encouraging activity. This combination has also been evaluated in a number of phase II studies in both first- (70~72) and second- (73) line treatment settings for advanced gastric cancer. The reported overall response rates were between 43 and 56%, with median survival durations between 8.6 and 10 months, which were comparable with results reported from studies using FAMTX, ECF and ELF. Except for the oxaliplatin-related neurotoxicity, 5-FU/folinic acid/oxaliplatin regimens have shown moderate to mild myelosuppression according to the dosage and schedule of 5-FU. Thus, the 5-FU/folinic acid/oxaliplatin combination is an active regimen, with acceptable toxicities, for the treatment of advanced gastric cancer. The combination of oxaliplatin/irinotecan also showed promising activity, with a favorable toxicity profile (74). Currently, a randomized multicenter study (REAL-2) is underway, with a two by two factorial design, to compare the efficacies of capecitabine with 5-FU, and oxaliplatin with cisplatin in the ECF regimen, for patients with advanced esophagogastric cancer. The interim analysis of the REAL-2 study showed good antitumor activity in favor of oxaliplatin and capecitabine, with a response rate of 52% in an EOX (epirubicin, oxaliplatin and capecitabine) regimen (75).
The combination of capecitabine, a promising oral 5-FU prodrug, and cisplatin has demonstrated an overall response rate and median survival of 55% and 10.1 months, respectively as a first-line treatment in previously untreated patients (76). This regimen was also active for the patients with relapsed gastric cancer after fluoropyrimidine-based adjuvant chemotherapy, with a response rate and median survival of 28% and 11.2 months, respectively (77). Two different combination schedules of docetaxel and capecitabine have been attempted for the treatment of advanced gastric cancer. In a weekly combination of docetaxel and capecitabine trial, fifty five patients were treated with docetaxel (36 mg/m2 intravenously), on days 1 and 8, and capecitabine (1,000 mg/m2 orally twice a day), on days 1~14, in a 3-week schedule until progression occurred. The overall response rate and median survival were 40.4% and 12.0 months, respectively (78). When docetaxel was administered every 3 weeks, at a dosage of 75 mg/m2, with capecitabine, at a dosage of 1,250 mg/m2, twice daily on days 1~14, a much higher response rate (60%) was reported, with a median survival of 10.5 months (79). However, this combination treatment showed a high incidence of stomatitis or hand-foot syndrome as the dose-limiting toxicities, which prevented continuous treatment.
The results of TS-1 containing combination chemotherapies have mainly been reported from Japanese case reports. TS-1, combined with either cisplatin or irinotecan, with various doses and schedules, has been reported by Japanese investigators. Only one report, showing a response rate for TS-1 plus cisplatin of 74% in phase I/II study, has been published in a peer review journal (80). Considering the high response rate and longer survival duration reported with capecitabine based combination chemotherapies and the high single agent activity of TS-1, the combination chemotherapies of TS-1 plus taxane, platinum or irinotecan may emerged as the new standard or reference regimens for the treatment of advanced gastric cancer.
Most combination chemotherapy regimens for the treatment of advanced gastric cancer have shown overall response rates in the range of 30 to 50%, usually in phase II studies. Despite the fact the median survival has remained significantly unchanged with the use of new regimens, some progress has been achieved in the treatment of advanced gastric cancer. It has been clearly shown that chemotherapy is better than the best supportive care alone, with respect to both the median survival and quality of life. Although, it has failed to translate into a survival gain, combination chemotherapy appears to be associated with significantly higher overall response rates than monotherapy. Recently, taxane has emerged as an attractive agent in combination with 5-FU and/or cisplatin. Taxane is also useful as a second-line treatment, agent due to its unique action and activity. Oral 5-FU prodrugs are replacing cumbersome 5-FU long-term infusions due to their convenience and superior toxicity profiles. Oxaliplatin could be an ideal alternative to the toxic cisplatin. However, the low complete response rate and short response duration are still the main obstacles in chemotherapy for advanced gastric cancer. It seems that the treatment of advanced gastric cancer using conventional chemotherapeutic agents has reached a plateau in efficacy, but further effort to find better combination chemotherapy regimens, in terms of toxicity profile and survival, still need to be pursued. We are waiting on the results of large phase III randomized clinical trials for the answers to these questions.
References
1. Shin HR, Jung KW, Won YJ, Park JG. 2002 annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004; 36:103–114.

2. Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000; 50:7–33. PMID: 10735013.

3. Glimelius B, Ekstrom K, Hoffman K, Grat W, Sjoden PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997; 8:163–168. PMID: 9093725.

4. Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995; 71:587–591. PMID: 7533517.

5. Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993; 72:37–41. PMID: 8508427.

6. Ajani JA. Standard chemotherapy for gastric carcinoma: Is it a myth? J Clin Oncol. 2000; 18:4001–4003.

7. Comis RL, Carter SK. A review of chemotherapy in gastric cancer. Cancer. 1974; 34:1576–1586. PMID: 4279136.

8. Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol. 2003; 21:54–59. PMID: 12506170.

9. Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, et al. phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993; 71:3813–3818. PMID: 8508349.
10. Johnson PW, Thompson PI, Seymour MT, Deasy NP, Thuraisingham RC, Slevin ML, et al. A less toxic regimen of 5-fluorouracil and high-dose folinic acid for advanced gastrointestinal adenocarcinomas. Br J Cancer. 1991; 64:603–605. PMID: 1911206.

11. Louvet C, de Gramont A, Demuynck B, Nordlinger B, Maisani JE, Lagadec B, et al. High-dose folinic acid, 5-fluorouracil bolus and continuous infusion in poor-prognosis patients with advanced measurable gastric cancer. Ann Oncol. 1991; 2:229–230. PMID: 2043494.

12. Arbuck SG, Douglass HO Jr, Trave F, Milliron S, Baroni M, Nava H, et al. A phase II trial of 5-fluorouracil and high-dose intravenous leucovorin in gastric carcinoma. J Clin Oncol. 1987; 5:1150–1156. PMID: 3498014.

13. Moertel CG, Lavin PT. Eastern Cooperative Oncology Group. Phase II-III chemotherapy studies in advanced gastric cancer. Cancer Treat Rev. 1979; 63:1863–1869.
14. Preusser P, Achterrath W, Wilke H, Lenaz L, Fink U, Heinicke A, et al. Chemotherapy of gastric cancer. Cancer Treat Rev. 1988; 15:257–277. PMID: 3071419.

15. Ajani JA, Fairweather J, Dumas P, Patt YZ, Pazdur R, Mansfield PF. Phase II study of Taxol in patients with advanced gastric carcinoma. Cancer J Sci Am. 1998; 4:269–274. PMID: 9689986.
16. Cascinu S, Graziano F, Cardarelli N, Marcellini M, Giordani P, Menichetti ET, et al. Phase II study of paclitaxel in pretreated advanced gastric cancer. Anticancer Drugs. 1998; 9:307–310. PMID: 9635920.

17. Ohtsu A, Boku N, Tamura F, Muro K, Shimada Y, Saigenji K, et al. An early phase II study of a 3-hour infusion of paclitaxel for advanced gastric cancer. Am J Clin Oncol. 1998; 21:416–419. PMID: 9708646.

18. Einzig AI, Neuberg D, Remick SC, Karp DD, O'Dwyer PJ, Stewart JA, et al. Phase II trial of docetaxel (Taxotere) in patients with adenocarcinoma of the upper gastrointestinal tract previously untreated with cytotoxic chemotherapy: the Eastern Cooperative Oncology Group (ECOG) results of protocol E1293. Med Oncol. 1996; 13:87–93. PMID: 9013471.

19. Mai M, Sakata Y, Kanamaru R, Kurihara M, Suminaga M, Ota J, et al. A late phase II clinical study of RP56976 (docetaxel) in patients with advanced or recurrent gastric cancer: a cooperative study group trial (group B). Gan To Kagaku Ryoho. 1999; 26:487–496. PMID: 10097745.
20. Taguchi T, Sakata Y, Kanamaru R, Kurihara M, Suminaga M, Ota J, et al. Late phase II clinical study of RP56976 (docetaxel) in patients with advanced/recurrent gastric cancer: a Japanese Cooperative Study Group trial (group A). Gan To Kagaku Ryoho. 1998; 25:1915–1924. PMID: 9797814.
21. Bang YJ, Kang WK, Kang YK, Kim HC, Jacques C, Zuber E, et al. Docetaxel 75 mg/m(2) is active and well tolerated in patients with metastatic or recurrent gastric cancer: a phase II trial. Jpn J Clin Oncol. 2002; 32:248–254. PMID: 12324575.

22. Tanizawa A, Fujimori A, Fujimori Y, Pommier Y. Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J Natl Cancer Inst. 1994; 86:836–842. PMID: 8182764.

23. Hsiang YH, Lihou MG, Liu LF. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989; 49:5077–5082. PMID: 2548710.
24. Kohne CH, Catane R, Klein B, Ducreux M, Thuss-Patience P, Niederle N, et al. Irinotecan is active in chemonaive patients with metastatic gastric cancer: a phase II multicentric trial. Br J Cancer. 2003; 89:997–1001. PMID: 12966415.

25. Lin-Shin L, Hecht J. A phase II trial of irinotecan in patients with advanced adenocarcinoma of the gastroesophageal (GE) junction. Proc Am Soc Clin Oncol. 2000; 19:289a. (abst 1130).
26. Futatsuki K, Wakui A, Nakao I, Sakata Y, Kambe M, Shimada Y, et al. CPT-11 Gastrointestinal Cancer Study Group. Late phase II study of irinotecan hydrochloride (CPT-11) in advanced gastric cancer. Gan To Kagaku Ryoho. 1994; 21:1033–1038. PMID: 8210254.
27. Ota K, Taguchi T, Kimura K. Report on nationwide pooled data and cohort investigation in UFT phase II study. Cancer Chemother Pharmacol. 1988; 22:333–338. PMID: 3139315.

28. Kim YH, Cheong SK, Lee JD, Park JS, Shin SW, Kim JS, et al. Phase II trial of Oral UFT and leucovorin in advanced gastric carcinoma. Am J Clin Oncol. 1996; 19:212–216. PMID: 8610653.

29. Ravaud A, Borner M, Schellens JH, Geoffrois L, Schoffski P, Wanders J, et al. UFT and oral calcium folinate as first-line chemotherapy for metastatic gastric cancer. Oncology (Huntingt). 1999; 13(7):Suppl 3. 61–63. PMID: 10442364.
30. Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998; 34:1274–1281. PMID: 9849491.

31. Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol. 2000; 45(4):291–297. PMID: 10755317.
32. Hong YS, Song SY, Lee SI, Chung HC, Choi SH, Noh SH, et al. A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol. 2004; 15:1344–1347. PMID: 15319239.

33. Kondo K, Chin K, Sakamoto J, Kojima H, Terashima M, Yamamura Y, et al. A multicenter phase II trial using 4-week cycles of capecitabine in advanced/metastatic gastric cancer (AGC). Proc Am Soc Clin Oncol. 2003; 22:321. (Abstr).
34. Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996; 7:548–557. PMID: 8862723.

35. Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998; 34:1715–1720. PMID: 9893658.
36. Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology. 2000; 58:191–197. PMID: 10765119.
37. Chollet P, Schoffski P, Weigang-Kohler K, Schellens JH, Cure H, Pavlidis N, et al. Phase II trial with S-1 in chemotherapy-naive patients with gastric cancer. A trial performed by the EORTC Early Clinical Studies Group (ECSG). Eur J Cancer. 2003; 39:1264–1270. PMID: 12763215.
38. Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol. 2003; 21:54–59. PMID: 12506170.

39. Wilke HJ, Van Cutsem E. Current treatments and future perspectives in colorectal and gastric cancer. Ann Oncol. 2003; 14(Suppl 2):ii49–ii55. PMID: 12810459.

40. Wils JA, Klein HO, Wagener DJ, Bleiberg H, Reis H, Korsten F, et al. Sequential high-dose methotrexate and fluorouracil combined with doxorubicin--a step ahead in the treatment of advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J Clin Oncol. 1991; 9:827–831. PMID: 2016625.

41. Preusser P, Wilke H, Achterrath W, Fink U, Lenaz L, Heinicke A, et al. Phase II study with the combination etoposide, doxorubicin, and cisplatin in advanced measurable gastric cancer. J Clin Oncol. 1989; 7:1310–1317. PMID: 2671287.

42. Lerner A, Gonin R, Steele GD Jr, Mayer RJ. Etoposide, doxorubicin, and cisplatin chemotherapy for advanced gastric adenocarcinoma: results of a phase II trial. J Clin Oncol. 1992; 10:536–540. PMID: 1548518.

43. Kelsen D, Atiq OT, Saltz L, Niedzwiecki D, Ginn D, Chapman D, et al. FAMTX versus etoposide, doxorubicin, and cisplatin: a random assignment trial in gastric cancer. J Clin Oncol. 1992; 10:541–548. PMID: 1548519.

44. O'Connell MJ. Etoposide, doxorubicin, and cisplatin chemotherapy for advanced gastric cancer: an old lesson revisited. J Clin Oncol. 1992; 10:515–516. PMID: 1548514.
45. Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000; 18:2648–2657. PMID: 10894863.

46. Cocconi G, Carlini P, Gamboni A, Gasperoni S, Rodino C, Zironi S, et al. Cisplatin, epirubicin, leucovorin and 5-fluorouracil (PELF) is more active than 5-fluorouracil, doxorubicin and methotrexate (FAMTX) in advanced gastric carcinoma. Ann Oncol. 2003; 14:1258–1263. PMID: 12881389.

47. Findlay M, Cunningham D, Norman A, Mansi J, Nicolson M, Hickish T, et al. A phase II study in advanced gastro-esophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF). Ann Oncol. 1994; 5:609–616. PMID: 7993836.

48. Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997; 15:261–267. PMID: 8996151.

49. Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002; 20:1996–2004. PMID: 11956258.

50. Lacave AJ, Baron FJ, Anton LM, Estrada E, DeSande LM, Palacio I, et al. Combination chemotherapy with cisplatin and 5-fluorouracil 5-day infusion in the therapy of advanced gastric cancer: a phase II trial. Ann Oncol. 1991; 2:751–754. PMID: 1801881.

51. Rougier P, Ducreux M, Mahjoubi M, Pignon JP, Bellefqih S, Oliveira J, et al. Efficacy of combined 5-fluorouracil and cisplatinum in advanced gastric carcinomas. A phase II trial with prognostic factor analysis. Eur J Cancer. 1994; 30A:1263–1269. PMID: 7999410.

52. Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, et al. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993; 71:3813–3818. PMID: 8508349.

53. Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, et al. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999; 15:295–301. PMID: 10023695.

54. Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol. 1999; 22:580–586. PMID: 10597742.
55. Kornek GV, Raderer M, Schull B, Fiebiger W, Gedlicka C, Lenauer A, et al. Effective combination chemotherapy with paclitaxel and cisplatin with or without human granulocyte colony-stimulating factor and/or erythropoietin in patients with advanced gastric cancer. Br J Cancer. 2002; 86:1858–1863. PMID: 12085176.

56. Ridwelski K, Gebauer T, Fahlke J, Kroning H, Kettner E, Meyer F, et al. Combination chemotherapy with docetaxel and cisplatin for locally advanced and metastatic gastric cancer. Ann Oncol. 2001; 12:47–51. PMID: 11249048.

57. Roth AD, Maibach R, Martinelli G, Fazio N, Aapro MS, Pagani O, et al. Swiss Group for Clinical Cancer Research (SAKK). European Institute of Oncology (EIO). Docetaxel (Taxotere)-cisplatin (TC): an effective drug combination in gastric carcinoma. Ann Oncol. 2000; 11:301–306. PMID: 10811496.

58. Schull B, Kornek GV, Schmid K, Raderer M, Hejna M, Lenauer A, et al. Effective combination chemotherapy with bimonthly docetaxel and cisplatin with or without hematopoietic growth factor support in patients with advanced gastroesophageal cancer. Oncology. 2003; 65:211–217. PMID: 14657594.

59. Roth AD, Maibach R, Fazio N, Cessa C, Stupp R, Morant R, et al. 5-Fluorouracil as protracted continuous intravenous infusion can be added to full-dose docetaxel (Taxotere)- cisplatin in advanced gastric carcinoma: a phase I-II trial. Ann Oncol. 2004; 15:759–764. PMID: 15111343.
60. Ajani JA, Fodor M, Van Cutsem E, Tjulandin S, Moiseyenko V, Cabral F, et al. Multinational randomized phase II trial of docetaxel (T) and cisplatin (C) with or without 5-fluorouracil (FU) in patients (pts) with advanced gastric or GE junction adenocarcinoma (AGC-AGEJC). Proc Am Soc Clin Oncol. 2000; 19:247a. (Abstr).
61. Ajani JA, VanCutsem E, Moiseyenko V, Tjulandin S, Fodor M, Majlis A, et al. Docetaxel (D), cisplatin, 5-fluorouracil compared to cisplatin (C) and 5-fluorouracil (F) for chemotherapy-naive patients with metastatic or locally recurrent, unresectable gastric carcinoma (MGC): Interim results of a randomized phase III trial (V325). Proc Am Soc Clin Oncol. 2003; 22:249. (Abstr).
62. Oh SC, Park KH, Choi IK, Yoon SY, Kim SJ, Seo JH, et al. Docetaxel (Taxotere), cisplatin, UFT, and leucovorin combination chemotherapy in advanced gastric cancer. Br J Cancer. 2005; 92:827–831. PMID: 15726097.

63. Lee SH, Kang WK, Park J, Kim HY, Kim JH, Lee SI, et al. Combination chemotherapy with epirubicin, docetaxel and cisplatin (EDP) in metastatic or recurrent, unresectable gastric cancer. Br J Cancer. 2004; 91:18–22. PMID: 15188010.

64. Pozzo C, Barone C, Szanto J, Padi E, Peschel C, Bukki J, et al. Irinotecan in combination with 5-fluorouracil and folinic acid or with cisplatin in patients with advanced gastric or esophageal-gastric junction adenocarcinoma: results of a randomized phase II study. Ann Oncol. 2004; 15:1773–1781. PMID: 15550582.

65. Bugat R. Irinotecan in the treatment of gastric cancer. Ann Oncol. 2003; 14(Suppl 2):ii37–ii40. PMID: 12810456.

66. Shirao K, Shimada Y, Kondo H, Saito D, Yamao T, Ono H, et al. Phase I-II study of irinotecan hydrochloride combined with cisplatin in patients with advanced gastric cancer. J Clin Oncol. 1997; 15:921–927. PMID: 9060529.

67. Boku N, Ohtsu A, Shimada Y, Shirao K, Seki S, Saito H, et al. Phase II study of a combination of irinotecan and cisplatin against metastatic gastric cancer. J Clin Oncol. 1999; 17:319–323. PMID: 10458249.

68. Shimada S, Yagi Y, Kuramoto M, Aoki N, Ogawa M. Second-line chemotherapy with combined irinotecan and low-dose cisplatin for patients with metastatic gastric carcinoma resistant to 5-fluorouracil. Oncol Rep. 2003; 10:687–691. PMID: 12684644.
69. Kollmannsberger C, Rick O, Derigs HG, Schleucher N, Schoffski P, Beyer J, et al. Activity of oxaliplatin in patients with relapsed or cisplatin-refractory germ cell cancer: a study of the German Testicular Cancer Study Group. J Clin Oncol. 2002; 20:2031–2037. PMID: 11956262.

70. Louvet C, Andre T, Tigaud JM, Gamelin E, Douillard JY, Brunet R, et al. Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol. 2002; 20:4543–4548. PMID: 12454110.

71. Chao Y, Yeh KH, Chang CJ, Chen LT, Chao TY, Wu MF, et al. Phase II study of weekly oxaliplatin and 24-h infusion of high-dose 5-fluorouracil and folinic acid in the treatment of advanced gastric cancer. Br J Cancer. 2004; 91:453–458. PMID: 15226770.

72. Al-Batran SE, Atmaca A, Hegewisch-Becker S, Jaeger D, Hahnfeld S, Rummel MJ, et al. Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol. 2004; 22:658–663. PMID: 14966088.

73. Kim DY, Kim JH, Lee SH, Kim TY, Heo DS, Bang YJ, et al. Phase II study of oxaliplatin, 5-fluorouracil and leucovorin in previously platinum-treated patients with advanced gastric cancer. Ann Oncol. 2003; 14:383–387. PMID: 12598342.

74. Souglakos J, Syrigos K, Potamianou A, Polyzos A, Boukovinas I, Androulakis N, et al. Combination of irinotecan (CPT-11) plus oxaliplatin (L-OHP) as first-line treatment in locally advanced or metastatic gastric cancer: a multicentre phase II trial. Ann Oncol. 2004; 15:1204–1209. PMID: 15277259.

75. Sumpter KA, Harper-Wynne C, Cunningham D, Oates J, Tebbutt N, Iveson T, et al. Randomised, multicenter phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric cancer: confirmation of dose escalation. Proc Am Soc Clin Oncol. 2003; 22:257. (Abstr).
76. Kim TW, Kang YK, Ahn JH, Chang HM, Yook JH, Oh ST, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. Ann Oncol. 2002; 13:1893–1898. PMID: 12453857.

77. Kang HJ, Chang HM, Kim TW, Ryu MH, Sohn HJ, Yook JH, et al. Phase II study of capecitabine and cisplatin as first-line combination therapy in patients with gastric cancer recurrent after fluoropyrimidine-based adjuvant chemotherapy. Br J Cancer. 2005; 92:246–251. PMID: 15655540.

78. Chun JH, Kim HK, Lee JS, Choi JY, Hwangbo B, Lee HG, et al. Weekly docetaxel in combination with capecitabine in patients with metastatic gastric cancer. Am J Clin Oncol. 2005; 28:188–194. PMID: 15803015.

79. Park YH, Ryoo BY, Choi SJ, Kim HT. A phase II study of capecitabine and docetaxel combination chemotherapy in patients with advanced gastric cancer. Br J Cancer. 2004; 90:1329–1333. PMID: 15054450.

80. Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, et al. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer. 2003; 89:2207–2212. PMID: 14676796.

Table 3
Randomized trials of combination chemotherapy in advanced gastric cancer
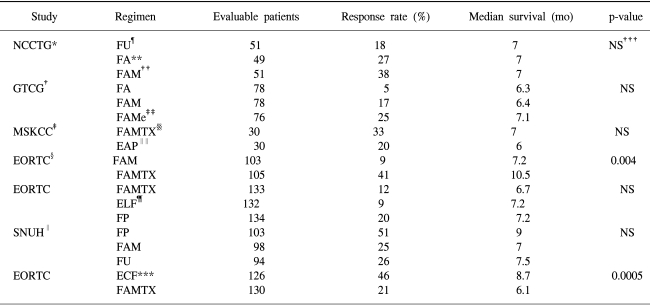
*North Central Cancer Treatment Group, †Gastrointestinal Tract Cooperative Group, ‡Memorial Sloan-Kettering Cancer Center, §European Organisation for Research and Treatment of Cancer, ∥Seoul National University Hospital, ¶5-fluorouracil, **5-fluorouracil/adriamycin, ††5-fluorouracil/adriamycin/mitomycin-C, ‡‡5-fluorouracil/adriamycin/methyl lomustine, §§5-fluorouracil/adriamycin/methotrexate; ∥∥etoposide/adriamycin/cisplatin, ¶¶etoposide/leucovorin/5-fluorouracil, ***epirubicin/cisplatin/5-fluorouracil, †††not significant.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download