Abstract
Purpose
Human papillomavirus (HPV) infection has a significant role in cervical carcinogenesis, and HPV oncoprotein E7 plays an important part in the formation and maintenance of cervical cancer. Interleukin-12 (IL-12) has been reported to induce a cellular immune response, and to suppress the tumor growth and the E7 production. Here we describe the use of adenoviral delivery of the HPV 16 E7 subunit (AdE7) along with adenoviral delivery of IL-12 (AdIL-12) in mice with HPV-associated tumors.
Materials and Methods
Mice were injected with TC-1 cells to establish TC-1 tumor, and then they were immunized with AdIL-12 and/or AdE7 intratumorally. The anti tumor effects induced by AdIL-12 and/or E7 were evaluated by measuring the size of the tumor. E7-specific antibody and INF-γ production in sera, and the T-helper cell proliferative responses were then measured. Cytotoxic T-lymphocyte (CTL) and T cell subset depletion studies were also performed.
Results
Combined AdIL-12 and AdE7 infection at the tumor sites significantly enhanced the antitumor effects more than that of AdIL-12 or AdE7 single infection. This combined infection resulted in regression of the 9 mm sized tumors in 80% of animals as compare to the PBS group. E7-specific antibody and INF-γ production in the sera, and the T-helper cell proliferative responses were significantly higher with coinfection of AdIL-12 and AdE7 than with AdIL-12 or AdE7 alone. CTL response induced by AdIL-12 and AdE7 in the coinjected group suggested that tumor suppression was mediated by mostly CD8+ and only a little by the CD4+ T cells.
IL-12 is known be a natural killer cell stimulatory factor and a cytotoxic lymphocyte maturation factor. It is a heterodimeric cytokine produced primarily by antigen presenting cells, including macrophages, dendritic cells and B cells; it is composed of two disulfide-links subunits, p35 (α chain) and p40 (β chain). Assembly of these subunits results in the biologically active p70 heterodimer (1~5). A previous report has shown that mouse IL-12 p40 homodimer is a potent IL-12 antagonist for cancer (5). Stimulation of IL-12 has several advantages for creating an immune response. For example, it enhances the cytolytic activity of a number of effector cells including T cells, natural killer (NK) cells, lymphokine activated killer (LAK) cells and macrophages; it increases proliferation of activated NK and T cells and it induces the production of cytokines such as IFN-γ. Subsequently, it stimulates the induction of Th1 cells that are critical for an effective host defense against intracellular pathogens; IL-12 up-regulates a number of cell surface molecules for the activation of differentiated T lymphocytes of both the CD4+ and CD8+ phenotype. It also inhibits IgE secretion, and acts as a synergistic factor for hematopoietic stem cells (6). Based on these potent immunomodulatory activities, IL-12 has been evaluated in several disease models for its potential roles against bacterial and parasitic infections, allergy and malignancies. Several therapeutic approaches have been studied in order to effectively deliver IL-12 into the target sites, and several vectors have been used. Recombinant protein or IL-12 secreting fibroblasts have been used (7,8), and viruses carrying IL-12 have also been studied such as adeno virus vector (7), retrovirus vector (8), herpes simplex virus vector (9), canarypox virus vector (10) and adeno associated virus (11), and a non-virus vector has also been studied (12). Naked IL-12 DNA using gold beads has been reported to be an effective tool against tumor and malignancy (12). For the administration of cytokine genes, non-viral vectors are known to be better than virus vectors. However, the titers of the delivered genes using a viral vector are better than that using non-viral vectors.
HPV is a double stranded DNA tumor virus and it is specific for epithelial cells including skin, respiratory mucosa and the genital tract (13). Persisting infection with more than ten high-risk genotypes of HPV has been casually linked to cervical cancer. HPV can be classified into the high risk group-HPV 16 and 18, and the low risk group-HPV 6 and 11. HPV 16 is the predominant viral type in cervical cancer patients and it carries two important oncogenes E6 and E7. Because the oncogenes E6 and E7 are consistently retained and expressed, the E6 and E7 oncogenes are an attractive target for gene therapy (13). Immunization with E7 antigen in different disease models has been studied (14,15) and these studies have demonstrated that CTLs (CTL, Cytotoxic T-cell lymphocytes) are likely to be the most effective immunological effector mechanisms. In this study, HPV 16 E7 is used as a model antigen because E7 is important in the induction and maintenance of cellular transformation and it is coexpressed in most HPV containing cervical cancers and their precursor lesions. Therefore, vaccines targeting E7 provide an opportunity to prevent and treat HPV-associated cervical cancer.
Our group has previously shown that codelivery of recombinant E7 protein and CpG-oligodeoxynucleotide (CpG-ODN), and a coinjection with adenovirus IL-12 and E7 protein induced a E7-specific CTL response resulting in an enhanced protective immune response against cervical cancer (16,17). In this study, we investigated the efficacy of codelivery of recombinant IL-12 and HPV 16 E7 carrying adenovirus against cervical cancer.
The Mouse lung cancer TC-1 cell line, which was derived from primary epithelial cells of C57BL/6 mice cotransformed with HPV-16 E6 and E7 and c-Ha-ras oncogenes (from the Cancer Research Center, Seoul National University, Korea), were cultured with the RPMI 1,640 media (Gibco BRL, Rocksville, MD) containing 10% fetal bovine serum (FBS) (Gibco BRL). Streptomycin/penicillin (Gibco BRL), L-glutamine (Gibco BRL), 2.2 mg/ml sodium bicarbonate (Sigma, St. Louis, MO) and 0.4 mg/ml G418 disulfate (Duchefa, Netherland) were added to the culture medium and the cells were maintained in a humidified environment at 37℃ and 5% CO2.
For the construction of AdE7, the full protein coding region of the HPV 16 E7 gene was reverse transcription-polymerase chain reaction (RT-PCR) amplified from a TC-1 cell line with the following pair of primers: sense primer, 5'-GGATCCACCATGCATGGACCTAAGGCA-3' and antisense primer, 5'-CTCGAGTGTTGCTTACTGCT-3'. E7 DNA fragments and the AdEasy™ vector system (Quantum, Montreal, Canada) were used for the production of AdE7. Briefly, the E7 DNA fragment was first cloned into the transfer vector. The resulting plasmid was then linearized with Pme I and cotransformed into E. coli BJ5183 together with AdEasy-1, the viral DNA plasmid. The pAdEasy-1 had its E1 and E3 deleted and its E1 function was supplemented in the 293A cells. The recombinants were selected with kanamycin and screened by restriction enzyme analysis. The recombinant adenoviruses were then cleaved with Pac I to expose their Inverted Terminal Repeats (ITR) and next they were transfected into QBI-293A cells to produce the viral particles, and then purified by double cesium chloride/ethidium bromide (CsCl) gradient ultracentrifugation (25,000 rpm, 4℃ 2 h) as previously described (18). The virus titers were also determined by plaque forming assay in 293 cells (19) and the expression levels of E7 were detected by western blot analysis. The AdIL-12 was kindly provided by Dr. Sung, Y.C. (POSTECH, Pohang, Korea). Control vector; AdLacZ cantaining a LacZ gene under the control of the cytomegalovirus promoter was previously used.
The AdE7 protein sample was separated on 12% SDS polyacrylamide gel, and then the separated proteins were electrophoretically transferred to a nitrocellulose membrane (Amersham, Piscataway, NJ). The blot was pre-equilibrated for 1 hour with TBST (10 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20) containing 2% BSA and then it was reacted with antiE7 monoclonal antibodies (Oncogene, Boston, MA) for 1 h at room temperature. After three washes with TBST, the membrane was incubated with antimouse IgG-HRP (Sigma) for 1 h at room temperature. The immuno-reactive protein bands were visualized using ECL detection reagents (Amersham).
Female C57BL/6 mice 4~6 weeks old were purchased from Daehan Biolink, Chungbuk, Korea. Mice were injected subcutaneously with 2×105 TC-1 tumor cells. When the tumor reached 8~9 mm in size, the tumor sites were immunized with a 100 µl volume of 5×108 plaque forming units (pfu) of AdIL-12, or AdLacZ. The sera were collected over time. The AdIL-12 infection doses had been tested and determined previously (16). The expression level of IL-12 was measured in triplicate using the Mouse IL-12 p70 Duo Set ELISA development kit (R & D Systems, Minneapolis, MN) according to manufacture's recommendation.
Preclinical evaluations were carried out in C57BL/6 mice by using the TC-1 tumor model (20). Female 4 to 6 weeks old C57BL/6 mice were purchased from Daehan Biolink, Korea. TC-1 cells (2×105 cells) were injected subcutaneously into the right flank of the mice. When the tumor reaches approximately 8~9 mm in mean diameter, AdLacZ, AdIL-12 and/or AdE7 were injected directly into the tumor at a final concentration of 5×108 pfu in a final volume of 100 µl of PBS. For the coimmunization with AdIL-12 and AdE7, AdIL-12 (5×108 pfu) was injected and then AdE7 (5×108 pfu) was also injected into the tumor site at the same time as the AdIL-12 injection, and a second set of injections with the above agents was performed 1 week after the first injection. The time schedule for the injection was reported previously (16). AdLacZ was used as a control. Mice were monitored over an 18 day period and the size of the tumors was measured in millimeters using a caliper. The results were recorded as mean diameters. Bars are indicated as standard deviations (SD).
For the detection of the relative levels of the E7-specific IgG subclasses, ELISA was performed as described previously (16). Briefly, recombinant E7 protein (1 µg/ml) was used as a coating antigen, and antimurine IgG1, IgG2a, IgG2b and IgG3 conjugated with HRP (Sigma) were substituted for the antimurine IgG-HRP. To determine the ELISA titer, equal volumes of sera were pooled from 6 mice in each group and then this was diluted 1:50 and reacted with E7 protein. The titers were determined by measuring the absorbance at 405 nm.
The T cell proliferation assay was performed as described previously. In brief, spleen cells were stimulated with E7 proteins at 0.5, 1, 5 and 10 µg/ml concentrations for 3 days. Then, [3H]-labeled thymidine (1 µCi/well) was added overnight. The cells were harvested the next day, and the cpm was counted using a β-counter (Perkin-Elmer, Boston, MA). The stimulation index was determined as [(experimental cpm - medium control cpm)/(media control cpm)].
A 5 h 51Cr release assay was performed. Briefly, splenocytes were stimulated for 5 days in the presence of 20 units/ml of interleukin 2 (R & D Systems) with TC-1 cells that had been previously treated for 3 h with mitomycin C (30 µg/ml). TC-1 target cells (104 per well) were labeled with 100 µCi/ml Na251CrO4 for 2 h and they were used to incubate with the stimulated splenocytes for 5 h at 37℃. One-hundred µl of supernatants were harvested and counted on a gamma counter (Perkin-Elmer). The percentage of specific lysis was determined as percent cytotoxicity±SD [(experimental release - spontaneous release)/(maximum release - spontaneous release)]×100%. Maximum release was determined by lysis of the target cells in 1% Triton X-100. An assay was not considered valid if the value for the spontaneous release counts was in excess of 20% of the maximum release value.
Expression levels of IFN-γ were measured by an ELISA using OptEIA™ Mouse IFN-γ kit (Pharmingen, California, CA) according to the manufacture's recommendation.
Depletion studies were performed as described previously (16,17). For in vivo cell depletion, anti-CD4 (clone GK1.5) and anti-CD8 (clone 2.43) ascites fluids were generated by injecting hybridoma cells (american Type Culture Collection, Manassas, VA) into pristane-primed nude mice interperitoneally One-hundred µl of ascites fluids were administered i.p. on days -3, 0 and 3 of the tumor challenge. Antibody treatment resulted in more than 98% depletion of the specific CD4+ and CD8+ T-cell subsets of the representative animals over a 3 week period. The depleted mice were subsequently immunized with AdIL-12 and/or AdE7 on day 0. The tumor size was then measured in millimeters using a caliper and the results were recorded as mean diameters.
Mice were immunized intratumorally with AdIL-12 or with AdLacZ, which used as a control, for 2 weeks and the time-dependent IL-12 expression levels in the animals' sera was measured by sandwich ELISA. As shown in Fig. 1 (A), the amount of IL-12 expression in the serum was at the highest level at day 1 after intratumoral AdIL-12 injection (6 ng/ml). A subsequently decreased level of IL-12 in the sera was observed till day 9. However, mice immunized with AdE7 or AdLacZ showed no expression of IL-12 in their sera.
To confirm the E7 protein expression in AdE7 transfected cells, CaSki cells that contain an integrated HPV type 16 genome (about 600 copies per cell) as well as sequences related to HPV-18 were infected together with a concentration of 100 MOI of AdE7 and AdLacZ. The expression level of E7 protein was detected by western blot analysis (Fig. 1B) and this data indicated that AdE7 transfected cells exhibited levels of E7 protein expression (lane 2) comparable with the AdLacZ transfected cells (lane 1). The E7 protein band in lane 2 was approximately 20 KDa.
To determine the antitumor effects of the codelivery of AdIL-12 and AdE7 against an established tumor in the murine cervical cancer model, mice injected with TC-1 tumor cells were immunized intratumorally with AdIL-12 or/and AdE7 and AdLacZ. The size of tumor was monitored over an 18 day period and it was measured in mm. As shown in Fig. 2, the AdIL-E7 immunized group showed only an 18% decrease; however, the AdIL-12 immunized group showed a 45% decrease and coinjection of AdIL-12 and AdE7 showed a significant 80% decrease in tumor growth as compared to the control and AdLacZ injection group.
To determine the effect of AdIL-12 and AdE7 on the induction of antigen-specific antibody responses, ELISA titers of the pooled serum were determined. The production patterns of IgG subclasses induced by the AdIL-12 and/or AdE7 are exhibited in Fig. 3. AdIL-12 and AdE7 codelivery enhanced E7-specific IgG subclass responses at a significantly higher level than the AdLacZ or PBS control at 4 weeks after the first immunization. The production of IgG1, IgG2a, IgG2b and IgG3 antibodies in the coimmunized group of AdIL-12 and AdE7 were higher than the antibody production from a single injection of AdIL-12 or AdE7.
T cell proliferation is a standard parameter to evaluate the potency of cell-mediated immunity. We measured the T cell proliferative response after coimmunization with AdIL-12 and AdE7 by stimulating splenocytes from the immunized animals in vitro with E7 proteins. As shown in Fig. 4 (A), we observed the significant enhancement of T cell proliferative responses with immunization in the AdIL-12 and AdE7 group and this was followed by the AdE7 and AdIL-12 single injection group. In contrast, the AdLacZ group showed little effects on the levels of T cell proliferative responses.
To determine whether coinjection of AdIL-12 and AdE7 could induce E7-specific CTL activity in vivo, we immunized animals with AdIL-12 and/or AdE7. As shown in Fig. 4 (B), coinjection with AdIL-12 and AdE7 alone induced CTL activities to a significant level. However, animals immunized with AdIL-12 or AdE7 alone showed little induction of CTL.
It was previously showed that E7 in the presence of IL-12 derived from a virus form elicited significant E7 specific CTL responses as compared to the control in vivo (16). Therefore, we also measured IFN-γ levels in the serum of murine cervical cancer model mice by ELISA in this study. AdIL-12 or AdE7 injection resulted in the highest increase of IFN-γ expression (43.3 pg/ml and 26.7 pg/ml, respectively) at day 1 only; however, coinjection of AdIL-12 and AdE7 resulted in an increased IFN-γ expression at day 1 (54.4 pg/ml) and the IFN-γ level remained high until day 7 (4 pg/ml). The expression level was higher than the AdIL-12, AdE7, AdLacZ or PBS immunized group (Fig. 5).
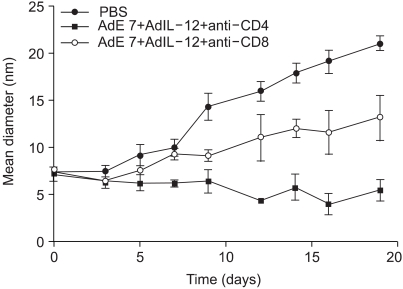
To understand the relative roles of the CD4+ or CD8+ Tcells for the antitumor immunity induced by AdIL-12 and AdE7 coinjection, the mice (n = 6 in each group) were injected with TC-1 cells. When the tumor size reached approximately 7~8 mm, the animals were depleted of CD4+ or CD 8+ T cells and then immunized with AdIL-12 and AdE7. Our current data, as shown in Fig. 6, exhibited similar patterns to our previous report (16,17), that the CD8+ T cell-depleted animal group showed a small delay, but there was formation of tumor, as compared with the PBS group. Moreover, the animals depleted of CD4+ T cells were completely protected from tumor growth.
Cervical cancer is caused in most cases by infection with a high-risk group of HPV. Both surgical and radiation therapies have been used with limited success in relapsing cases of cervical cancer. Therefore, development of new therapeutic strategies is important and this has been attempted by many researchers. Previous findings have shown the positive effects of IL-12 on the immune response; injection of AdIL-12 into murine adenocarcinoma, fibrosarcoma and colon cancers has demonstrated complete tumor suppression (21). Tumor specific antigens are capable of inducing strong T cell mediated protective immunity against tumors when the antigens are presented by antigen presenting cells to the CD4+ and CD8+ T cells. Because HPV 16 E7 antigen is highly immunogenic and frequently expressed in cervical cancer, several alternate candidate vectors or methodologies have been attempted for developing therapeutic and prophylactic vaccines using E7 antigen in animal disease models. For example, the production of E7 proteins (21), DNA vaccine encoding the translocation domain of a bacterial toxin conjugated with E7 (22), E7 carrying the different viral vectors (13) and gene-based vaccination of DCs with E7 antigen (24) were found to be effective in cervical cancer. These reports showed that E7 antigen used as a vaccine could induce CD8+ T lymphocytes activity that was responsible for the antitumor effects noted during the immune response. For these reasons, our group attempted, in a previous study, to protect against tumor growth by coinjecting E7 proteins and AdIL-12 into a tumor site, and we showed that 40% of the animals were completely tumor-free and the remaining animals also displayed far reduced tumor sizes over time (16). In this report, intratumoral injection of AdIL-12 in mice induced IL-12 expression that was detected systemically in vivo, and the IL-12 expression lasted a long time. The lysates from E7 cells revealed a protein band with a size approximately ~20 KDa, which was a molecular size bigger than we expected. Our productin of adenovirus was probably easier than the protein production.
We investigated the antitumor effect of AdIL-12 and AdE7 coinjection as compared to injection with either AdIL-12 or AdE7 alone, and we measured the tumor size. We used a dose of 2×105 TC-1 given subcutaneously and this dose led to the rapid development of tumors within 10 days. The tumors commonly reached the required 8~9 mm size. We have provided the first evidence that intratumoral injection of AdE7 with AdIL-12 induced protective immunity against infection by HPV 16. In more detail, adenoviral infection conferred protection for up to 45% or 18% of mice immunized with the AdIL-12 or AdE7, respectively. Furthermore, when the mice were immunized with both AdIL-12 and AdE7, tumor suppression was shown in about 80% of mice. This was more than a 35% increase in tumor suppression rate as compared with a single infection of AdIL-12. Our previous study showed only 40% tumor regression by coinjection of E7 proteins and AdIL-12, and the remaining animals displayed far reduced tumor sizes over time (16). Therefore, the differences of the ratio of antitumor effects between our previous study and this study were significant, and we can say that AdIL-12 and AdE7 coinfection were a powerful delivery system to suppress tumor as compare to AdIL-12 + E7 protein in mice with HPV-associated tumor.
The E7-specific immune response was also confirmed by the measurement of antibody production in this study. The production pattern of the IgG subclasses give an indication of the Th1 vs. Th2 nature of the induced immune responses; IgG and IgE represent the Th2 response and IgG2a represents the Th1 response. However, our results showed that IgG1, IgG2a and IgG2b antibody productions were increased by coinjection of AdIL-12 and AdE7 as compared with coinjection of AdIL-12 or AdE7 alone. The OD value of IgG3 appeared to be quite low and it was not correlated with the other antibody's data. Our data showed that IgG1, IgG2b and IgG3 production by coinjection of AdIL-12 and AdE7 were statistically significant. Therefore, the increased IgG production (Th2 & Th1) levels suggested that it was E7-specific humoral immunity. Antibody plays little or no role in established HPV infections; however, it contributes to the prevention of reinfection with the same HPV type.
Similarly, T cell proliferations were significantly enhanced by a coinjection with AdIL-12 and AdE7 more than for AdIL-12 or AdE7 alone in a manner similar to the antibody responses we observed. However, the CTL activities, when the mice were immunized with AdIL-12 and/or AdE7, were only significantly induced by a coinjection with AdIL-12 and AdE7. There was little induction of CTL activities by the delivery of either AdIL-2 or AdE7 alone. This was in agreement with previous findings (16), supporting that the codelivery of AdIL-12 and AdE7 could be useful for the induction of E7-specific CTL responses in vivo.
Previous findings have shown it is likely that IFN-γ might play an important role in controlling tumor growth (25), and IL-12 as well as IL-12 derived IFN-γ might be involved in the E7-specific CTL immune response (16). Therefore, our data suggested that the coinjection of AdIL-12 and AdE7 modulated the Th1 response, and the modulation level was higher as compared to the AdIL-12 or AdE7 single injection group.
We additionally evaluated the involvement of a possible role for CD4+ vs. CD8+ T cells for protecting animals from the TC-1 tumor challenge. The CD8+ T cell-depleted animal group showed a delayed, but complete loss of protection against tumor, suggesting that CD8+ T cells are the major effector T-cell population. When the CD4+ T cells were depleted, protection from tumor was observed, as compared with the nondepleted animal group. These data suggested a contributing minor role for CD4+ T cells and a major role for CD8+ T cells in antitumor immunity. Taken together, these data support that AdIL-12 and AdE7 coinjection can induce protection from tumor growth mostly through effects of CD8+ T cells and a little by the effects of CD4+ T cells in vivo.
Only vaccines containing expressed viral antigens have been proven to have long term advantages as compare with other non-viral antigens, although non-viral vaccines these have had limited disease applications (13).
In conclusion, coinjection of AdIL-12 and AdE7 showed effective suppression of tumor growth in TC-1 tumor cell-injected mice. E7 antigen specific antibody production and IFN-expression were appeared to be correlated with the CTL immune response. These results suggested that the induction of IL-12 and E7 mediated CTL immune responses will provide us with useful tools as a possible immune therapy modality against HPV-associated cervical cancer.
ACKNOWLEDGEMENTS
We thank Dr. T-C Wu (The John's Hopkins Medical Institutions, Baltimore, MD), for providing TC-1 cells and Dr. Y.C. Sung (Postech, Pohang, Korea) for providing AdIL-12.
References
1. Gately MK, Desai BB, Wolitzky AG, Quinn PM, Dwyer CM, Podlaski FJ, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol. 1991; 147:874–882. PMID: 1713608.
2. Gubler U, Chua AO, Schoenhaut DS, Dwyer CM, McComas W, Motyka R, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991; 88:4143–4147. PMID: 1674604.

3. Trinchieri G, Scott P. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res Immunol. 1995; 146:423–431. PMID: 8839141.

4. Yoon C, Johnston SC, Tang J, Stahl M, Tobin JF, Somers WS. Charged residues dominate a unique interlocking topography in the heterodimeric cytokine interleukin-12. EMBO J. 2000; 19:3530–3541. PMID: 10899108.

5. Vandenbroeck K, Alloza I, Gadina M, Matthys P. Inhibiting cytokines of the interleukin-12 family: recent advances and novel challenges. J Pharm Pharmacol. 2004; 56:145–160. PMID: 15005873.

6. Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, et al. Natural killer cell stimulatory factor (interleukin-12 (IL-12)) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4 producing Th cells. J Exp Med. 1993; 177:1199–1204. PMID: 8096238.
7. Siders WM, Wright PW, Hixon JA, Alvord WG, Back TC, Wiltrout RH, et al. T cell and NK cell-independent inhibition of hepatic metastases by systemic administration of an IL-12-expressing recombinant adenovirus. J Immunol. 1998; 160:5465–5474. PMID: 9605149.
8. Tahara H, Zeh HJ 3rd, Storkus WJ, Pappo I, Watkins SC, Gubler U, et al. Fibroblasts genetically engineered to secrete interleukin-12 can suppress tumor growth and induce antitumor growth and induce anti-tumor immunity to a murine melanoma in vivo. Cancer Res. 1994; 54:182–189. PMID: 7903204.
9. Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci USA. 2000; 97:2208–2213. PMID: 10681459.

10. Puisieux I, Odin L, Poujol D, Moingeon P, Tartaglia J, Cox W, et al. Canarypox virus-mediated interleukin 12 gene transfer into murine mammary adenocarcinoma induces tumor suppression and long-term antitumoral immunity. Hum Gene Ther. 1998; 9:2481–2492. PMID: 9853515.

11. Paul D, Qazilbash MH, Song K, Xu H, Sinha BK, Liu J, et al. Construction of a recombinant adeno-associated virus (rAAV) vector expressing murine interleukin-12 (IL-12). Cancer Gene Ther. 2000; 7:308–315. PMID: 10770641.

12. Rakhmilevich AL, Janssen K, Turner J, Culp J, Yang NS. Cytokine gene therapy of cancer using gene gun technology: superior antitumor activity of interleukin-12. Hum Gene Ther. 1997; 8:1303–1311. PMID: 9295125.

13. Liu DW, Tsao YP, Kung JT, Ding YA, Sytwu HK, Xiao X, et al. Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 peptide DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer. J Virol. 2000; 74:2888–2894. PMID: 10684306.

14. Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996; 347:1523–1527. PMID: 8684105.

15. Boursnell ME, Rutherford E, Hickling JK, Rollinson EA, Munro AJ, Rolley N, et al. Construction and characterisation of a recombinant vaccinia virus expressing human papillomavirus proteins for immunotherapy of cervical cancer. Vaccine. 1996; 14:1485–1494. PMID: 9014288.

16. Ahn WS, Bae SM, Kim TY, Kim TG, Lee JM, Namkoong SE, et al. A therapy modality using recombinant IL-12 adenovirus plus E7 protein in a human papillomavirus 16 E6/E7-associated cervical cancer animal model. Hum Gene Ther. 2003; 14:1389–1399. PMID: 14577920.

17. Kim TY, Myoung HJ, Kim JH, Moon IS, Kim TG, Ahn WS, et al. Both E7 and CpG-oligodeoxynucleotide are required for protective immunity against challenge with human papillomavirus 16 (E6/E7) immortalized tumor cells: involvement of CD4+ and CD8 T+ cells in protection. Cancer Res. 2002; 62:7234–7240. PMID: 12499264.
18. Bae SM, Kim YW, Yoon JH, Yoo JY, Seo YS, Nam SL, et al. Cell-specific growth inhibition of human cervical cancer cell by recombinant adenovirus p53 in vitro and in vivo. Cancer Res Treat. 2003; 35:181–190.

19. Mitchell MF, Hamada K, Sastry KJ, Sarkar A, Tortolero-Luna G, Wharton JT, et al. Transgene expression in the rhesus cervix mediated by an adenovirus expressing beta-galactosidase. Am J Obstet Gynecol. 1996; 174:1094–1101. PMID: 8623835.
20. Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996; 56:21–26. PMID: 8548765.
21. Gambotto A, Tuting T, McVey DL, Kovesdi I, Tahara H, Lotze MT, et al. Induction of antitumor immunity by direct intratumoral injection of a recombinant adenovirus vector expressing interleukin-12. Cancer Gene Ther. 1999; 6:45–53. PMID: 10078963.

22. Fernando GJ, Murray B, Zhou J, Frazer IH. Expression, purification and immunological characterization of the transforming protein E7, from cervical cancer-associated human papillomavirus type 16. Clin Exp Immunol. 1999; 115:397–403. PMID: 10193409.

23. Hung CF, Cheng WF, Hsu KF, Chai CY, He L, Ling M, et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 2001; 61:3698–3703. PMID: 11325841.
24. Tillman BW, Hayes TL, DeGruijl TD, Douglas JT, Curiel DT. Adenoviral vectors targeted to CD40 enhance the efficacy of dendritic cell-based vaccination against human papillomavirus 16-induced tumor cells in a murine model. Cancer Res. 2000; 60:5456–5463. PMID: 11034088.
25. Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997; 15:749–795. PMID: 9143706.
Fig. 1
(A) Production of IL-12 in serum after delivery of AdIL-12 in mice. Animals were injected subcutaneous ly with 2×105 TC-1 tumor cells. When the tumor reached 9 mm in size, the tumor site was immunized with 5×108 pfu of AdIL-12 or AdLacZ. The sera were collected over the time as indicated. The level of IL-12 in sera was measured in triplicate using sandwich ELISA. (B) CaSki cells were infected with AdE7 at 100 MOI. The total cell extract was made and then subjected to western blot analysis for the E7 antigen. E7 antigen was showed as the band of 20 KDa in lane 2.
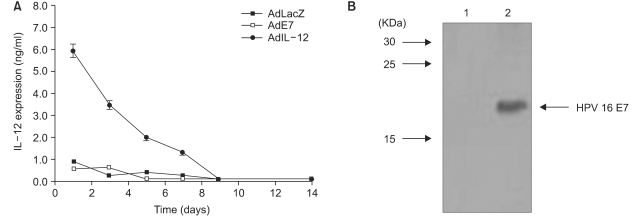
Fig. 2
Effects of AdIL-12 and AdE7 codelivery on tumor growth over time. Each group of mice (n = 6) was inoculated subcutaneously with 2×105 TC-1 cells. When the tumor size reached 9 mm, the animals were immunized intratumorally with PBS, AdLacZ (5×108 pfu), AdIL-12 (5×108 pfu) and/or AdE7 (5×108 pfu). The size of tumor was monitored over an 18 day period. Mean diameter of tumor size is shown. Values and bars represent the mean and standard deviation (SD) of the tumor sizes, respectively.

Fig. 3
Induction of E7-specific IgG isotypes by injection with AdIL-12 and/or AdE7. Each group of mice (n = 6) was immunized interperitoneally with 5×108 pfu of AdIL-12 and/or AdE7. The mice were bled at 2 and 4 weeks after the virus injection. (AD): the sera of the 2 and 3 weeks groups were diluted to 1:50 and reacted with E7 protein in ELISA. Absorbance was measured at 405 nm. *, statistically significant at p<0.05 using student's T test compared with AdE7 alone; bars, ±SD.
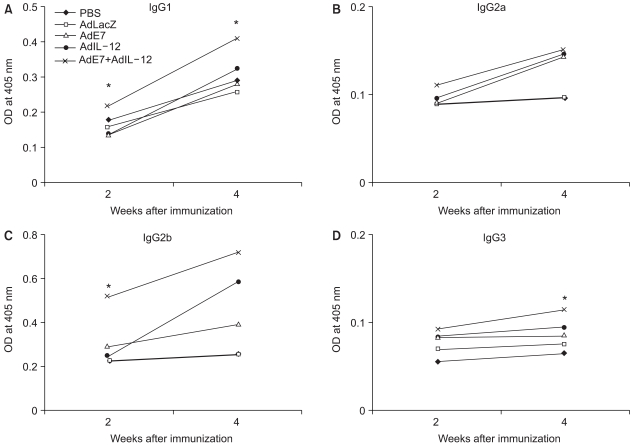
Fig. 4
Induction of E7-specific T-cell proliferation and CTL responses by injection with AdIL-12 and/or AdE7. For the T cell proliferation assay (A), splenocytes from immunized mice were stimulated in vitro with 0.5, 1, 5 and 10 µg/ml AdIL-12 and/or AdE7. After 3 days of stimulation, the cells were harvested and then the cpm was counted. Samples were assayed in triplicate. For CTL assay (B), splenocytes were stimulated in vitro with mitomycin C-treated TC-1 cells. The specific cytolytic activity was tested against TC-1 cells in a 51Cr release assay. The results represent the mean specific lysis values from individual, representative mice tested at the indicated Effector: Target (E:T) ratio. The experiments were repeated two more times with similar results. *, statistically significant at p <0.05 using student's T test compared with negative controls. **, statistically significant at p <0.05 using student's T test compared with AdE7 alone.

Fig. 5
Production levels of IFN-γ from serum of mice immunized with AdIL-12 or/and AdE7. The mice were immunized with AdIL-12 or AdE7, or a combination of AdIL-12 and AdE7. Samples were assayed in triplicate. Values and bars represent the mean of released IFN-γ concentrations and standard deviation (SD), respectively.
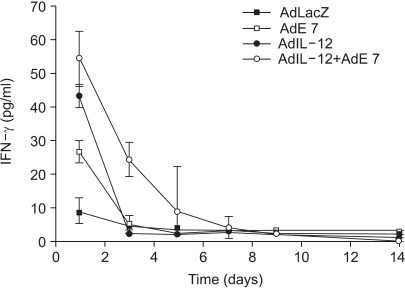
Fig. 6
Effects of T-cell subsets on tumor growth. The mice (n = 6 in each group) were injected with TC-1 cells. When the tumor size reached approximately 7~8 mm, the animals were depleted of CD4+ or CD 8+ T cells and then immunized with AdIL-12 and AdE7. The size of tumors was measured for 3 weeks. Values and bars represent the mean and standard deviation (SD) of the tumor size, respectively.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download