Abstract
Purpose
Gastric cancer is one of the most prevalent cancers worldwide. 5-fluorouracil (5-FU) and cisplatin are the most commonly used drugs for the treatment of gastric cancer. However, a significant number of tumors often fail to respond to chemotherapy.
Materials and Methods
To better understand the molecular mechanisms underlying drug resistance in gastric cancer the gene expression in gastric cancer cells, which were either sensitive or resistant to 5-FU and cisplatin, were examined using cDNA microarray analysis. To confirm the differential gene expression, as determined using the microarray, semiquantitative RT-PCR was performed on a subset of differentially expressed cDNAs.
Results
69 and 45 genes, which were either up-regulated (9 and 22 genes) or down-regulated (60 and 25 genes), were identified in 5-FU- and cisplatin-resistant cells, respectively. Several genes, such as adaptor-related protein complex 1 and baculoviral IAP repeat-containing 3, were up-regulated in both drug-resistant cell types. Several genes, such as the ras homolog gene family, tropomyosin, tumor rejection antigen, protein disulfide isomerase-related protein, melanocortin 1 receptor, defensin, cyclophilin B, dual specificity phosphatase 8 and hepatocyte nuclear factor 3, were down-regulated in both drugresistant cell types.
Conclusion
These findings show that cDNA microarray analysis can be used to obtain gene expression profiles that reflect the effect of anticancer drugs on gastric cancer cells. Such data may lead to the assigning of signature expression profiles of drug-resistant tumors, which may help predict responses to drugs and assist in the design of tailored therapeutic regimens to overcome drug resistance.
Gastric cancer is one of the leading causes of cancer death in the world (1). Although the incidence of gastric cancer is increasing, many patients still remain inoperable and are treated with palliative chemotherapy. 5-Fluorouracil and cisplatin are the most commonly used anticancer drugs in the treatment of gastrointestinal tract cancer, including gastric cancer. However, a significant number of tumors often fail to respond to chemotherapy. Tumors usually consist of a mixed population of malignant and normal cells. The effectiveness of treatment will depend on the response of individual cells to the drug. Although treatment kills sensitive cells, the remaining resistant cells continue developing, and form clinically resistant tumors. Resistance phenotypes, reflecting cell defense to environmental modifications, can be a major obstacle in cancer therapy. Resistance mechanisms are commonly divided into two broad categories: intrinsic resistance and induced resistance. Several mechanisms of drug resistance in tumors are understood and include the overexpression of the multidrug resistance gene, the multidrug resistance-associated protein and increased DNA repair (2~4). Mechanisms underlying the development of intrinsic drug resistance are not thoroughly understood, and may involve expression of multiple genes during tumor progression, while acquired resistance may be associated with drug selection during chemotherapy. It is essential to understand the mechanisms underlying resistance to chemotherapy in order to better treat tumors with anticancer drugs.
DNA microarray technology has enabled simultaneous analysis of expression of thousands of tumor cell genes (5~9). To better understand the molecular mechanisms underlying drug resistance in gastric cancer cells, we used cDNA microarray analysis to study differential gene expression profiles in gastric cancer cells that were either sensitive or resistant to 5-FU and cisplatin. The cells used in this study were derived from the SNU638 gastric cancer cell line (10).
The human gastric cancer cell line SNU638 was cultured in RPMI medium, containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100µg/ml streptomycin and 20 mM glutamine, in a 5% CO2 humidified air atmosphere at 37℃. Cells were treated continuously with stepwise-increased concentrations of 5-FU and cisplatin every 3 weeks, up to final concentrations of 200~800 µg/ml. The resultant 5-FU- and cisplatin-resistant cells (SNU638-F2 and SNUcis800, respectively) were each selected from one culture dish and further subcultured. These cell lines showed 2,117- and 20-fold acquired resistances to 5-FU and cisplatin, respectively (9).
A Human 10K cDNA microarray (GaiaGene Inc.) was used, which consisted of 10,336 spots, including UniGEM-V clones (Incyte Pharmaceuticals) and housekeeping genes, with yeast DNAs as negative controls. The UniGEM-V clones contain 9,182 unique and sequence-verified cDNA elements mapped to 8,353 UniGene Homo sapien annotated clusters (11).
Total RNA was extracted using Trizol reagent (Life Technologies). Probes were labeled using the amino allyl cDNA labeling kit (Ambion), as follows: 20 µg total RNA (12 µl) was combined with oligo(dT) primer (50 µM, 1 µl), incubated at 70℃ for 10 min, and then kept at room temperature. This primer/RNA solution was added to an RT mix, consisting of 2 µl 10x RT buffer, 1 µl RNase inhibitor (10 units/µl), 1 µl dNTP mix (10 mM dATP, dCTP and dGTP), 1 µl AAdUTP mix (3 mM dTTP and 3 mM amino allyl dUTP) and 2 µl MMLV reverse transcriptase (200 units/µl,), and incubated at 42℃ for 2 h. RNA was then hydrolyzed with NaOH (1 M, 4 µl) at 65℃ for 15 min. The solution was neutralized with HEPES (1 M, 10 µl) and the cDNA recovered by ethanol precipitation using sodium acetate (3 M, 3.4 µl) and ethanol (100%, 100 µl). The cDNA pellet was resuspended in 4.5 µl Coupling Buffer (Ambion) followed by the addition of 2.5 µl nuclease-free water and 3 µl dye (NHS-ester Cy3 or Cy5, 2.22 mM in DMSO). The resulting solution was mixed by gentle vortexing, and the briefly centrifuged. The sample tubes were wrapped in aluminum foil and incubated at room temperature for one hour in a dark room. The labeling reaction was stopped by the addition of 6 µl 4 M hydroxylamine hydrochloride, the tubes vortexed, briefly centrifuged, and incubated for 15 min at room temperature in the dark. To the solution, 70 µl nuclease-free water was added, after which the dye-labeled cDNA was purified using NucAway spin columns (Ambion). Purified, labeled cDNA was ethanol precipitated, pelleted by brief centrifugation and then resuspended in 10 µl nuclease-free water.
For prehybridization, the cDNA microarray was placed in a 50 ml conical tube containing prehybridization solution (5x SSC, 0.1% SDS and 1% bovine serum albumin) and incubated at 55℃ for 1 h. The Cy3- and Cy5-labeled products were combined, and 8 µg poly(dA) (Pharmacia), 4 µg Escherichia coli tRNA (Sigma Chemical), 10 µg Human Cot1 DNA (Life Technologies) and EasyHyb hybridization solution (U-Vision Biotech) added, to a final volume of 60 µl. The probe was denatured at 100℃ for 2 min, the debris cleared by centrifugation, and the probe solution placed onto the microarray slide, with a coverslip, in a hybridization chamber (TeleChem International). Hybridization was performed in a 55℃ water bath for 12~16 h. After a quick rinse in 0.1x SSC, the slides were then washed in 2x SSC/0.2% SDS at room temperature for 5 min and then 0.1x SSC/0.2% SDS at 55℃ for 5 min, and then spun dry (500 rpm) for 5 min.
The two fluorescent images (Cy3 and Cy5) were scanned separately using a GMS 418 Array Scanner (Affymetrix) and analyzed using the MAAS program (GaiaGene; http//www.gaiagene.com). Yeast DNA was spotted onto each slide to determine the background signal intensity. Values at least 2-fold above that of the background were considered significant. To filter out unreliable data, spots with signal-to-noise ratios below three were disregarded. Each experiment was performed three times.
From each sample, 5 µg total RNA was reverse-transcribed for single-stranded cDNA, using 1 µM oligo-dT primer, with Superscript II reverse transcriptase (Life Technologies, Inc.). Each single-stranded cDNA was diluted for subsequent PCR amplification by monitoring the GAPDH as a quantitative control. Each PCR was carried out in a 50 µl volume of 1x PCR buffer for 5 minutes at 95℃ for the initial denaturation, followed by 25~35 cycles at 95℃ for 45 seconds, 56℃ for 45 seconds and 72℃ for 1 min in a Perkin-Elmer Cycler 9600 (Perkin-Elmer Applied Biosystems, Foster, CA). The sequences of the primers used for RT-PCR were as follows: βactin forward, 5'-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3', reverse, 5'-CTA GAA GCA TTG CGG TGG ACG ATG GAG GG-3'; follistatin forward, 5'-AAG AGC AGC CAG AAC TGG AA-3', reverse, 5'-ACA GAC AGG CTC ATC CGA CT-3'; keratin 7 forward, 5'-ACT TCG AGA TCG CCA CCT AC-3', reverse, 5'-CCT CAG AAT GAG GCT GCT TT-3'; transmembrane protein 3 (1~8 U) forward, 5'-GCT GGA CAC CAT GAA TCA AA-3', reverse, 5'-ATA GGC CTG GAA GAT CAG CA-3'; protein complex 1 mu 2 subunit forward, 5'-TTG AGC ACT TCA TGC CTT TG-3', reverse, 5'-TGA CCA GCA GGT TGA CAG AC-3'; baculoviral IAP repeat-containing 3 forward, 5'-CAA CAG ATC TGG CAA AAG CA-3', reverse, 5'-GAA ACC ACT TGG CAT GTT GA-3'; pregnancy-induced growth inhibitor forward, 5'-CCT CAG ACC CTG TCC TCA TC-3', reverse, 5'-CAG GAT CCA CTG CAA AGT CA-3'; MnSOD forward, 5'-GTT GGC CAA GGG AGA TGT TA-3', reverse, 5'-CTG ATT TGG ACA AGC AGC AA-3' and cytochrome p450 forward, 5'-ACC CGA GAC ACC ATT TTC AG-3', reverse, 5'-TGA GGT CGA TAT CCT TTG GG-3'.
cDNAs obtained from 5-FU- or cisplatin-sensitive and -resistant SNU638 sublines were separately labeled with Cy5 and Cy3, respectively, and hybridized to the 10,336-spot gene microarray. To filter out unreliable data and identify genes with significantly different expression, two 'cutoff' analysis methods were employed, whereby spots with a signal-to-noise ratio below three, or with a signal intensity less than the negative control spots, were not investigated further. After this filtering, genes showing expression ratios greater than or equal to 2-fold increases or decreases were selected.
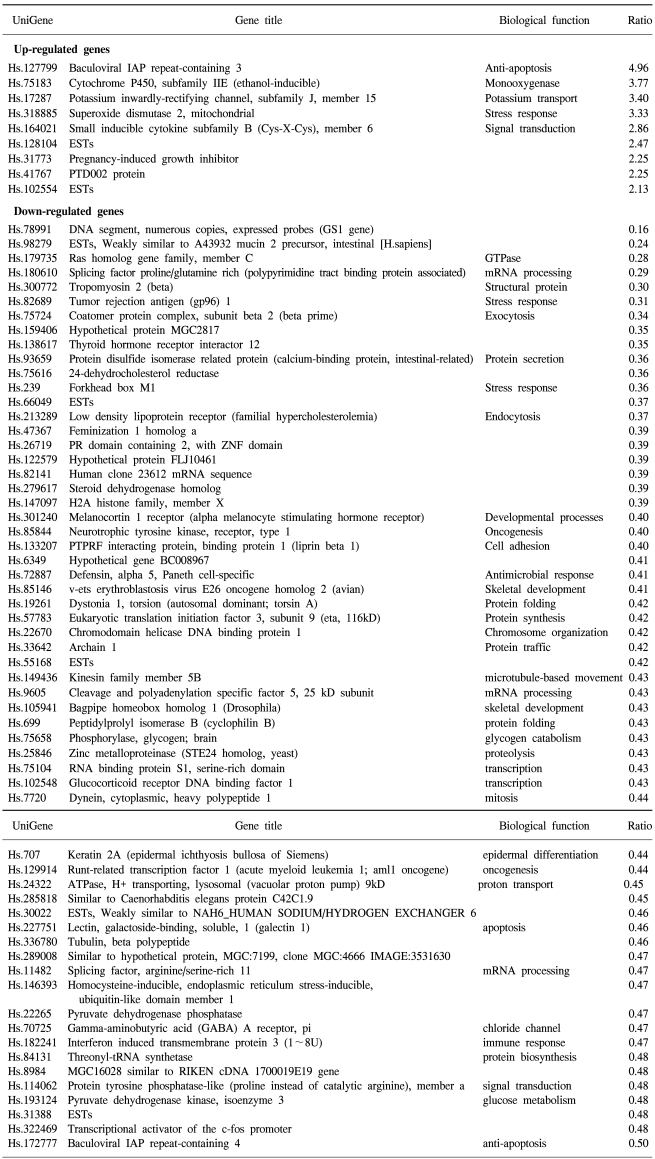
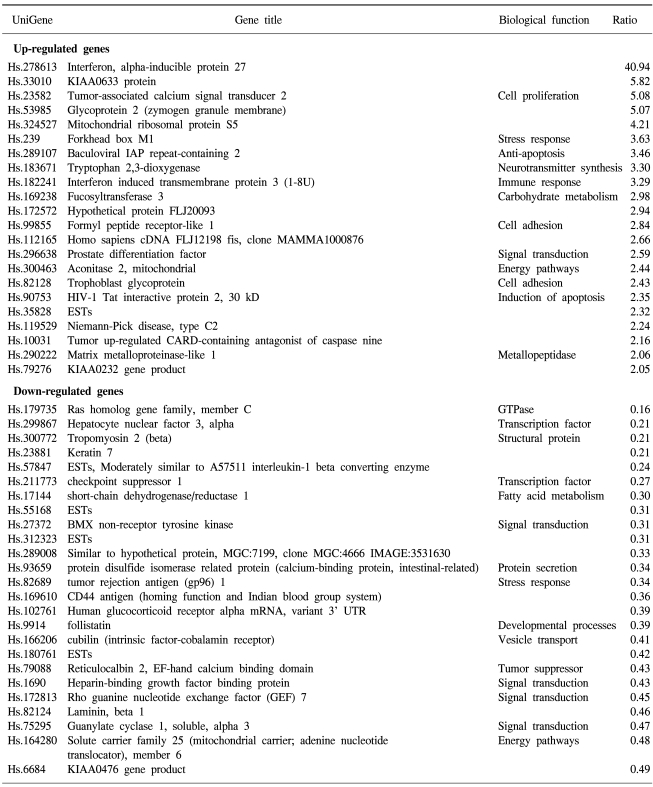
69 and 45 genes were identified that showed either up-regulated (9 and 22 genes) or down-regulated (60 and 25 genes) expression in 5-FU- and cisplatin-resistant gastric cancer cells, respectively. In the 5-FU-resistant cells, of the up-regulated spots, 7 were known genes and 2 were expressed sequence tags (ESTs), while of the down-regulated spots, 55 were known genes and 5 were ESTs (Table 1). In the cisplatin-resistant cells, the up-regulated spots included 21 known genes and 1 EST, while the down-regulated spots included 21 known genes and 4 ESTs (Table 2). Several EST clones were identified in both drug-resistant lines. The identification of some uncharacterized EST clones showing differential expression was promising, as such clones may prove to play important roles in characterizing gene expressions and the regulation in chemotherapy-resistant gastric cancer cells.
Seven of the 9 genes showing up-regulated expressions in the 5-FU-resistant gastric cancer cells have known function (Table 1). Of particular interest was the up-regulation of the baculoviral IAP repeat-containing gene, which is linked to apoptosis resistance. G-protein, inwardly-rectifying the potassium channel 1 (GIRK1) gene, was also up-regulated. Among the chemokines, the small inducible cytokine B subfamily gene showed increased expression, as did the cytochrome p450 gene, which encodes an enzyme known to play a central role in oxidative metabolism of a wide range of compounds. MnSOD, the product of the superoxide dismutase 2 (SOD2) gene, was also positively regulated. Genes showing down-regulated expressions in the 5-FU-resistant cells included those coding for tropomyosin 2, runt-related transcription factor, tubulin and the tumor rejection antigen. Similarly, the ras homolog gene family was expressed at lower levels.
Cisplatin-resistant gastric cancer cells showed increased expression of genes involved in apoptosis resistance, including the baculoviral IAP repeat and tumor up-regulated CARD genes. There was also increased expression of the signal transduction genes prostate differentiation factor and formyl peptide receptor-like 1. Genes involved in cell adhesion and invasion were also up-regulated, including the trophoblast glycoprotein and matrix metalloproteinase-like 1 genes. Genes showing lower expressions included those coding for structural proteins, such as tropomyosin 2, laminin, keratin 7 and protein disulfide isomerase-related protein.
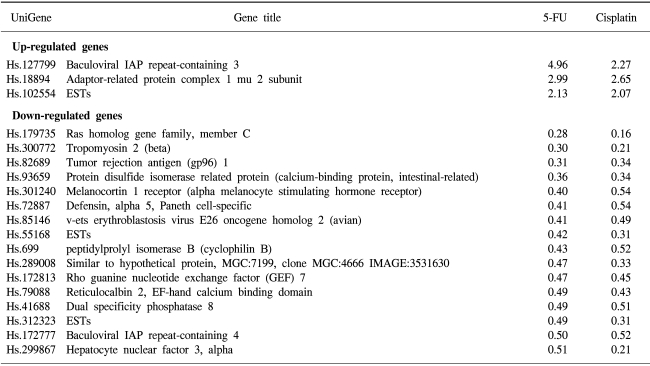
The expressions of two genes were up-regulated in both types of drug-resistant gastric cancer cells, namely the adaptor-related protein complex 1 and the baculoviral IAP repeat-containing 3 genes. A large number of identical genes were down-regulated in both drug resistant cell lines, including the ras homology gene family, tropomyosin, tumor rejection antigen, protein disulfide isomerase-related protein, melanocortin 1 receptor, defensin, avian, cyclophilin B, Rho guanine nucleotide exchange factor 7, reticulocalbin 2, dual specificity phosphatase 8 and hepatocyte nuclear factor 3 genes (Table 3). Some genes showed discordant expressions between the two cell lines. Tryptopan 2,3-dioxygenase, formyl peptide receptor-like 1, prostate differentiation factor, interferon alpha-inducible protein 27, interferon-induced transmembrane protein 3, forkhead box M1, tumor up-regulated CARD-containing antagonist of caspase nine and galectin 1 genes were down-regulated in the 5-FU-resistant cells, but up-regulated in the cisplatin- resistant cells. In contrast, follistatin, short-chain dehydrogenase/reductase 1 and pregnancy-induced growth inhibitor were up-regulated in the 5-FU-resistant cells, but down-regulated in the cisplatin-resistant cells.
To confirm the differential gene expression determined using the microarray, semiquantitative RT-PCR was performed on a subset of differentially expressed cDNAs. The RT-PCR analysis was used to examine the expressions of seven up-regulated genes (follistatin, keratin 7, protein complex 1 mu 2 subunit, baculoviral IAP repeat-containing 2, pregnancy-induced growth inhibitor, MnSOD and cytochrome p450) and one down-regulated gene (transmembrane protein 3) in the 5-FU-resistant cells. Similarly, the RT-PCR analysis was used to determine the expressions of four up-regulated (transmembrane protein 3, protein complex 1 mu 2 subunit, baculoviral IAP repeat-containing 2 and pregnancy-induced growth inhibitor) and four down-regulated (follistatin, keratin 7, MnSOD and cytochrome p450) genes in the cisplatin-resistant cells. The patterns of the gene expressions determined using RT-PCR analysis were found to be very similar to those using microarray analysis (Figs. 1, 2).
Resistance to chemotherapy remains a serious problem in the treatment of gastric cancers. We established a 5-FU-resistant subline, SNU638-F2, derived from the SNU638 human gastric adenocarcinoma cell line. SNU638-F2 was 2,117-fold more resistant to 5-FU than the parent cells. Similarly, from the same parent cells, a SNUcis800 subline was created which was 800-fold more resistant to cisplatin. To better understand the molecular mechanisms underlying 5-FU and cisplatin resistance in SNU638 cells, the mRNA expression levels profiles were examined using cDNA microarray analysis, and many genes identified whose expression were either up- or down-regulated compared to their chemosensitive counter parts.
The present study examined differential gene expressions in 5-FU-resistant cells. The most prominent finding was the altered expressions of apoptosis resistance-associated genes, such as IAP repeat-containing genes. The key effectors of apoptosis are caspases, which are cystein proteases that cleave after aspartate residues. The inhibitor of the apoptosis family of proteins prevents cell death by binding to and inhibiting active caspases (12). Thus, the inhibition of apoptosis may play a key role in chemotherapy resistance in gastric cancer cells. MnSOD, the product of the superoxide dismutase 2 (SOD2) gene, is one of the major cellular defenses against oxidative stress (13) and was found to be up-regulated in this study. This finding was consistent with a previous report that showed an elevated MnSOD level in 5-FU-resistant gastric cancer cells (10). The inwardly-rectifying potassium current was recently found in small cell lung cancer cells (14), and the overexpression of the G-protein inwardly-rectifying potassium channel in primary breast carcinomas was correlated with axillary lymph node metastasis (15). The G-protein inwardly-rectifying potassium channel is activated during ischemia, which promotes cellular survival and may be essential for cell protection against chemotherapy (16). A number of cytochrome p450 enzymes are known to metabolize a variety of anticancer drugs, with the usual consequence of cytochrome p450 metabolism being detoxification of the drug. In the present study, the cytochrome p450 gene expression was up-regulated in 5-FU-resistant gastric cancer cells, which was consistent with other data that suggested increased expressions of the human cytochrome p450 genes, CYP1A1 and CYP1B1, decrease the cell sensitivity to the cytotoxic effects of anticancer drugs (17,18).
Although cisplatin is one of the most widely used chemotherapeutic agents, its effectiveness is limited by both intrinsic and acquired resistance. The mechanisms of resistance thus far identified include the impaired uptake of the drug, increased efflux, intracellular detoxification (by glutathione for example), tolerance to cisplatin-DNA adducts and increased repair of DNA damage (19,20). The details of the biochemical steps involved in these processes have not been fully elucidated, and little information is available on how these disparate mechanisms coordinate with each other. The cisplatin-resistant gastric cancer cells were found to show elevated expressions of apoptosis resistance-associated genes, such as the IAP repeat-containing and tumor up-regulated human caspase recruitment domain (CARD)-containing genes. This was also the case in 5-FU-resistant cells, suggesting apoptosis resistance might be an important mechanism in drug resistance. Another interesting finding was the up-regulation of many genes involved in cell adhesion and invasion, such as matrix metalloproteinase-like 1, formyl peptide receptor-like 1 and trophoblast glycoprotein.
Los et al (21) used Affymetrix's oligonucleotide HuFL6800 array (Affymetrix, Santa Clara, CA) to monitor the mRNA expression profiles in five different pairs of cisplatin-sensitive and -resistant ovarian cancer cell lines. When the gene expression profiles of the cisplatin-resistant cell pairs were compared using hierarchical clustering, all five cisplatin-resistant sublines clustered with their corresponding parental cell line. However, the number of genes commonly expressed in any combination of two pairs was small, and disappeared when more than three pairs were compared. These data suggest the cisplatin-resistance phenotype is multi-factorial, which was apparent in the changes in the mRNA abundance accompanying the resistance phenotype in the various cisplatin-resistance pairs.
Kudoh et al (22) recently analyzed the gene expression profiles in doxorubicin-induced and doxorubicin-resistant MCF-7 cells using cDNA microarray analysis. The biochemical functions of the differentially expressed genes were diverse, including transcription factors, protein kinases and phosphatases, cell cycle regulators, proteases, apoptotic and anti-apoptotic factors, as well as a large number of metabolic genes. They also compared the expression profiles between doxorubicin-resistant and cisplatin-resistant MCF-7 cells. Although a subset of about 40 genes overlapped, these genes were distinct from those that showed different expression in the doxorubicin-resistant compared to doxorubicin-induced cells, demonstrating that the signature profiles of the doxorubicin- and the cisplatin-resistant cells were different. Similarly, in the present study, few genes were commonly up- or down-regulated in the 5-FU-resistant and cisplatin-resistant gastric cancer cells.
Watts et al (23) searched for differentially-expressed genes between a human multiple myeloma cell line and its doxorubicin-selected multidrug resistant counterpart. They found that doxorubicin selection produced multiple defects in the genes involved in apoptosis signaling pathways. Similarly, in the present study, apoptosis resistance-associated genes were found to be up-regulated in both the 5-FU- and cisplatin-resistant cell lines. These data indicate that changes in the gene expressions reducing apoptosis in response to death-inducing stimuli may contribute to the multidrug resistance phenotype, and such changes may be therapeutic targets for the treatment of gastric cancer.
The cDNA microarray will speed the discovery and progress in the understanding of cellular and subcellular resistance processes. The results from the present study suggest that the increased expressions of anti-apoptotic genes might be crucial for the acquisition of drug resistance by gastric cancer cells; thus, could be of use as therapeutic targets. Specific gene-mediated drug resistance warrants further investigation with regard to its likely therapeutic benefits.
References
1. Neugut AI, Hayek M, Howe G. Epidemiology of gastric cancer. Semin Oncol. 1996; 23:281–291. PMID: 8658212.
2. Gottesman MM, Pastan I, Ambudkar SV. P-glycoprotein and mutidrug resistance. Curr Opin Genet Dev. 1996; 6:610–617. PMID: 8939727.
3. Loe DW, Deeley RG, Cole SP. Biology of the multidrug resistance-associated protein, MRP. Eur J Cancer. 1991; 32A:945–957. PMID: 8763335.

4. Chaney SG, Sancar A. DNA repair: enzymatic mechanisms and relevance to drug response. J Natl Cancer Inst. 1996; 88:1346–1360. PMID: 8827012.

5. DeRisi J, Penland L, Brown PO, Bittner ML, Meltzer PS, Ray M, et al. Use of a cDNA microarray to analyse gene expression patterns in human cancer. Nat Genet. 1996; 14:457–460. PMID: 8944026.

6. DeRisi JL, Iyer VR, Brown PO. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997; 278:680–686. PMID: 9381177.

7. Marton MJ, DeRisi JL, Bennett HA, Iyer VR, Meyer MR, Roberts CJ, et al. Drug target validation and identification of secondary drug target effects using DNA microarrays. Nat Med. 1998; 4:1293–1301. PMID: 9809554.

8. Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999; 283:83–87. PMID: 9872747.

9. Ahn MJ, Lee KH, Ahn JI, Yu DH, Lee HS, Choi JH, et al. The differential gene expression profiles between sensitive and resistant breast cancer cells to adriamycin by c DNA microarray. Cancer Res Treat. 2004; 36:43–49.
10. Chung YM, Park S, Park JK, Kim Y, Kang Y, Yoo YD. Establishment and characterization of 5-fluorouracil-resistant gastric cancer cells. Cancer Lett. 2000; 159:95–101. PMID: 10974411.

11. Lee KH, Chang MY, Ahn JI, Yu DH, Jung SS, Chio JH, et al. Differential gene expression in retinoic acid-induced differenti ation of acute promyelocyteic leukemia cells, NB4 and HL-60 cells. Biochem Biophys Res Commun. 2002; 296:1125–1133. PMID: 12207890.
12. Verhagen AM, Coulson EJ, Vaux DL. Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol. 2001; 2:REVIEWS3009. PMID: 11516343.
13. Drane P, Bravard A, Bouvard V, May E. Reciprocal down-regulation of p53 and SOD2 gene expression: implication in p53 mediated apoptosis. Oncogene. 2001; 20:430–439. PMID: 11313974.
14. Sakai H, Shimizu T, Hori K, Ikari A, Asano S, Takeguchi N. Molecular and pharmacological properties of inwardly rectifying K+ channels of human lung cancer cells. Eur J Pharmacol. 2002; 435:125–133. PMID: 11821018.

15. Stringer BK, Cooper AG, Shepard SB. Overexpression of the G-protein inwardly rectifying potassium channel 1 (GIRK1) in primary breast carcinomas correlates with axillary lymph node metastasis. Cancer Res. 2001; 61:582–588. PMID: 11212253.
16. Crawford RM, Budas GR, Jovanovic S, Ranki HJ, Wilson TJ, Davies AM, et al. M-LDH serves as a sarcolemmal K(ATP) channel subunit essential for cell protection against ischemia. EMBO J. 2002; 21:3936–3948. PMID: 12145195.

17. McFadyen MC, McLeod HL, Jackson FC, Melvin WT, Doehmer J, Murray GI. Cytochrome p450 CYP1B1 protein expression: a novel mechanism of anticancer drug resistance. Biochem Pharmacol. 2001; 62:207–212. PMID: 11389879.
18. Brockdorff BL, Skouv J, Reiter BE, Lykkesfeldt AE. Increased expression of cytochrome p450 1A1 and 1B1 genes in antiestrogen-resistant human breast cancer cell lines. Int J Cancer. 2000; 88:902–906. PMID: 11093812.

19. Los G, Muggia FM. Platinum resistance. Experimental and clinical status. Hematol Oncol Clin North Am. 1994; 8:411–429. PMID: 8040146.

20. Oldenburg J, Begg AC, van Vugt MJ, Ruevekamp M, Schornagel JH, Pinedo HM, et al. Characterization of resistance mechanisms to cis-diamminedichloroplatinum (II) in three sublines of the CC531 colon adenocarcinoma cell line in vitro. Cancer Res. 1994; 54:487–493. PMID: 8275486.
21. Los G, Yang F, Samimi G, Manorek G, Guerorguieva IM, Howell S, et al. Using mRNA expression profiling to determine anticancer drug efficacy. Cytometry. 2002; 47:66–71. PMID: 11774355.

22. Kudoh K, Ramanna M, Ravatn R, Elkahloun AG, Bittner ML, Meltzer PS, et al. Monitoring the expression profiles of doxorubicin-induced and doxorubicin-resistant cancer cells by cDNA microarray. Cancer Res. 2000; 60:4161–4166. PMID: 10945624.
23. Watts GS, Futscher BW, Isett R, Gleason-Guzman M, Kunkel MW, Salmon SE. cDNA microarray analysis of multidrug resistance: doxorubicin selection produces multiple defects in apoptosis signaling pathways. J Pharmacol Exp Ther. 2001; 299:434–441. PMID: 11602652.
Fig. 1
Semi-quantitative RT-PCR analysis of the gene expressions in 5-FU-resistant cells. The left panel shows the RT-PCR results. The right panel indicates the relative mRNA ratios of the indicated genes. (A) The expressions of seven genes are shown to be up-regulated by the microarray analysis; follistatin, keratin 7, protein complex 1 mu 2 subunit, baculoviral IAP repeat-containing 2, pregnancy-induced growth inhibitor, MnSOD and cytochrome p450. (B) The expression of one gene is shown to be down-regulated by the microarray analysis; transmembrane protein 3.
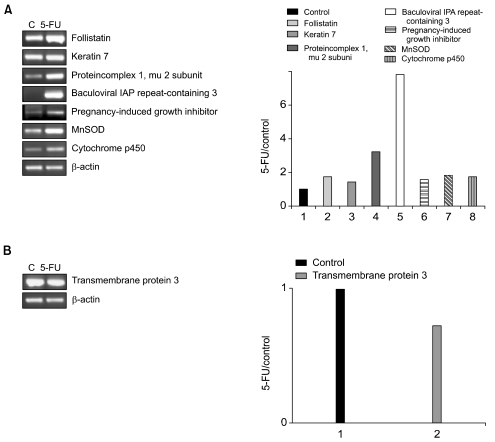
Fig. 2
Semi-quantitative RT-PCR analysis of the gene expressions in cisplatin-resistant cells. The left panel shows the RT-PCR results. The right panel indicates the relative mRNA ratios of the indicated genes. (A) The expressions of four genes are shown to be up-regulated by the microarray analysis; transmembrane protein 3, protein complex 1 mu 2 subunit, baculoviral IAP repeat-containing 2 and pregnancy-induced growth inhibitor. (B) The expressions of four genes are shown to be down-regulated by the microarray analysis; follistatin, keratin 7, MnSOD and cytochrome p450.

Table 1
Differential gene expressions in 5-FU-resistant gastric cancer cells, as determined by DNA microarray analysis (as compared to 5-FU-sensitive gastric cancer cells)




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download