Abstract
Purpose
We prospectively conducted a multi-center, open-label, randomized phase II trial to compare the efficacy and safety of docetaxel plus cisplatin (DC) and etoposide plus cisplatin (EC) for treating advanced stage non-small cell lung cancer (NSCLC).
Materials and Methods
Seventy-eight previously untreated patients with locally advanced, recurrent or metastatic NSCLC were enrolled in this study. The patients received cisplatin 75 mg/m2 on day 1 and either docetaxel 75 mg/m2 on day 1 or etoposide 100 mg/m2 on days 1 to 3 in the DC or EC arm, respectively, every 3 weeks.
Results
The objective response rate was 39.4% (15/38) and 18.4% (7/38) (p=0.023) in the DC and EC arms, respectively. The median time to progression (TTP) was 5.9 and 2.7 months (p=0.119), and the overall survival was 12.1 and 8.7 months (p=0.168) in the DC and EC arms, respectively. The prognostic factors for longer survival were an earlier disease stage (stage III, p=0.0095), the responders to DC (p=0.0174) and the adenocarcinoma histology (p=0.0454). The grades 3 and 4 toxicities were similar in both arms, with more febrile neutropenia (7.9% vs. 0%) and fatigue (7.9% vs. 0%) being noted in the DC arm.
Go to : 
Non-small cell lung cancer (NSCLC) is a common malignancy throughout the world, and it is one of the leading causes of cancer-related deaths. At the time of the initial diagnosis, 60~70% of patients with NSCLC have locally advanced or metastatic disease (1). Those patients with advanced disease who receive the best supportive care will survive for a few months, but approximately 90% of them will not survive beyond one year (2).
The use of chemotherapy for advanced NSCLC has recently become customary. Randomized trials and a series of large-scale meta-analyses have demonstrated that in comparison with supportive care, chemotherapy results in significant, although modest improvement for survival, symptoms and the quality of life (QoL) for the patients with advanced NSCLC (3~7).
Over the past decade, a number of new agents like paclitaxel, docetaxel, gemcitabine, vinorelbine, irinotecan, tirapazamine and carboplatin have been introduced for the treatment of NSCLC. The combination of cisplatin with these new agents is considered to be active treatment options for patients suffering with locally advanced or metastatic NSCLC (3,8,9).
Docetaxel and platinum compounds are monotherapeutic agents that are used to treat NSCLC (8~10). Docetaxel is a semi-synthetic taxoid derived from Taxus baccata, and it has exhibited activity even against platinum-resistant disease (10). Indeed, second-line docetaxel monotherapy has shown a significant survival benefit and improvement in the QoL as compared with the best supportive care for previously treated NSCLC patients (10,11). Docetaxel has been recommended by the American Society of Clinical Oncology as the standard second-line therapy for advanced NSCLC (12). Phase II studies have demonstrated the synergistic activity of docetaxel combined with cisplatin (DC), and this combination of agents has shown a predictable and manageable safety profile, with neutropenia being the main observed toxicity (13,14). A recent phase III study comparing DC and docetaxel plus carboplatin with combined vinorelbine plus cisplatin as first-line therapies for patients with advanced NSCLC showed that both the docetaxel-platinum combinations were better tolerated than was the combination of vinorelbine-cisplatin (15). In addition, DC resulted in a more favorable response rate and survival, and DC also demonstrated a consistently better QoL.
Before the newer chemotherapeutic agents were introduced, combined regimens of cisplatin with a vinca alkaloid or epipodophylotoxin were most commonly used to treat advanced NSCLC (16,17). The etoposide-cisplatin (EC) combination has produced a response rate of 30% (range: 17~69%), but there was no proven survival benefit over the best supportive care (16,17). Prior to the introduction of the newer anti-cancer agents, the EC combination was the standard treatment in Korea for patients with advanced NSCLC (18~20).
We prospectively conducted this phase II trial to compare the efficacy and safety of DC and EC for the treatment of locally advanced, recurrent or metastatic NSCLC.
Go to : 
This open-label, randomized, prospective, multi-center phase II trial was conducted at 7 institutions in Korea. In accordance with the declaration of Helsinki, the study protocol was approved by the relevant ethical committees of each participating institution, and a written informed consent was obtained from all patients.
The eligibility criteria included histologically or cytologically proven, unresectable, locally advanced stage IIIB and/or stage IV NSCLC. No prior palliative chemotherapy was permitted for the patients with advanced disease. Recurrent disease was defined as evident tumor progression after surgical resection or radiation treatment. The patients in this study were required to be at least 18 years of age, have a Karnofsky performance status (PS) of 80% or more and a life expectancy of at least 12 weeks, and the presence of at least one uni-dimensionally measurable lesion was mandatory. Additional eligibility criteria included adequate bone marrow reserves (a white blood cell count ≥3×109/L, platelets ≥100×109/L, hemoglobin ≥100g/L and a hematocrit ≥30%), and also adequate liver and renal function (creatinine <1.5 times the upper limit of normal).
Patients were excluded if they had any active infection, any uncontrolled central nervous system metastasis requiring emergency radiotherapy and/or corticosteroids, serious concomitant systemic disorders, a second primary malignancy (except in situ carcinoma of the cervix or non-melanomatous skin cancers) or any severe cardiovascular disease. Patients with peripheral neuropathy grade 2, according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) Version 2.0, were also excluded from the study. Any patients who were pregnant or breast-feeding were excluded from the study.
The patients were randomized to receive either the DC or EC chemotherapeutic regimen by using a randomized drawing from an envelope, and these regimens were randomly assigned to the patients at a 1:1 ratio. In the DC arm, the patients received docetaxel 75 mg/m2, and this was immediately followed by cisplatin 75 mg/m2; both drugs were administered as 1-hour intravenous (i.v.) infusions on day 1. Oral dexamethasone 8 mg (or an equivalent corticosteroid) was administered twice a day for 3 days beginning a day prior to each docetaxel infusion so as to prevent any anaphylactic reaction and/or fluid retention that would be caused by docetaxel. In the EC arm, the patients were given etoposide 100 mg/m2 as a 1-hour i.v. infusion on days 1 to 3, and they were given cisplatin 75 mg/m2 as 1-hour i.v. infusion on day 1. Cisplatin was infused with adequate hydration, diuretics and mannitol. Chemotherapy was repeated every 3 weeks in both arms and it was continued until there was objective evidence of disease progression, until a patient withdrew consent for further treatment or in the cases of early withdrawal from the trial because of an unacceptable adverse event or non-compliance with the trial protocol. Prophylactic use of granulocyte colony-stimulating factor (G-CSF) or antibiotics was not permitted. In the event of hematological and severe non- hematological toxicities that occurred at any time during treatment, the patients were treated by adjusting the dose of the chemotherapeutic agents. With regard to the hematological toxicity, a complete blood cell count with the differential and platelet counts were performed on each day of treatment. During the course of treatment, two dose reductions for each chemotherapeutic agent were allowed in the event of grades 3/4 hematological or non-hematological toxicities. All the patients were assessed in the follow-up phase for their disease progression and thereafter until death.
The clinical response evaluation procedure included all the laboratory or imaging studies that had shown abnormal findings prior to treatment. Tumor responses were assessed every two cycles by performing simple chest X-ray and CT scans of the chest and/or abdomen along with bone scans unless there was obvious evidence of disease progression after one cycle of therapy. The treatment responses were evaluated using the WHO criteria. Briefly, a complete response (CR) was defined as the absence of disease at all previously known tumor sites for at least four weeks. A partial response (PR) was defined as a 50% reduction in the sum of the products of the perpendicular diameters of all measurable lesions, and this response lasted at least four weeks. Progressive disease (PD) was defined as either a 25% increase in the area of any one lesion over the prior measurement, or the development of one or more new lesions. Stable disease (SD) was defined as any change in the previous lesion that did not fit into either the PR or PD categories. All the patients were evaluated for adverse reactions after the first dose of therapy according to the NCI-CTC grading system, version 2.
The sample size calculation was based on superiority testing for the response rate. When assuming that the treatment groups were allocated an equal number of subjects and that comparisons were based on the response rate, the null hypothesis of an equal response rate in the two groups was tested against the alternative hypothesis of a higher response rate in the DC arm. Assuming a response rate of 14% in the EC arm and 41% in the DC arm, according to a review of the literature, the correct sample size was calculated to be 33 subjects. This allowed for detection of the targeted alternative hypothesis with at least 80% power and a one-sided 5% level of significance.
The intent-to-treat (ITT) population included all the patients who were randomly assigned and treated, and who had undergone at least one disease assessment with using the same lesion imaging procedure that was used at the study's baseline. Per protocol analysis was performed on those subjects who were eligible and assessable for response without them having incurred any major protocol deviations. The primary analysis was based on the ITT population.
The primary efficacy variable, i.e., the objective response (OR), was defined as the best overall response for the CR or PR with using the WHO criteria. Differences in the OR between the treatment arms were assessed with using one-sided 5% Chi-square tests. The results were presented in terms of the estimated difference in the response rates and the p value. Disease control was defined as the best tumor response for the CR, PR or SD that was confirmed and sustained for 4 weeks or longer.
The Overall Survival (OS) was calculated from the date of enrollment to the date of death or the date when the patient was last known to be alive. The time to the progression (TTP) of disease was calculated from the date of enrollment to the date of progression or death; data for the patients who were alive and relapse-free were censored as of the date of the last known follow-up visit. The OS and TTP were estimated with using the Kaplan-Meier method and these parameters were compared in the two treatment arms with using the log-rank test. The median TTP and the 95% confidence interval (CI) were also estimated. A Cox proportional hazard regression model was used to further explore the observed differences and to identify the baseline factors that may independently predict patient survival.
Go to : 
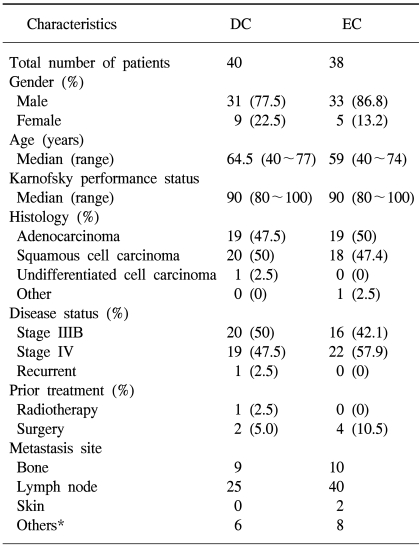
Between April 2000 and March 2002, a total of 78 previously untreated patients were identified and randomized. Of the 78 patients who were recruited for the study, 76 of them received chemotherapy and so they were included in the safety and efficacy analysis. Of the 78 patients, 2 (DC, n=2) were ineligible for analysis because of withdrawal of consent and proven brain metastasis, respectively. Seventy-six patients were treated in either the DC (n=38) or the EC (n=38) arm. Among the 38 treated patients in each arm, 4 patients in the DC arm and 5 in the EC arm were excluded from the response evaluation due to their discontinuation of chemotherapy after one cycle; this was attributable to consent withdrawal (DC, n=2; EC, n=3), lost follow-up (DC, n=1; EC, n=1) or other reasons (DC, n=1; EC, n=1). Thirty-four patients in the DC arm and 33 in the EC arm finally constituted the per-protocol population. All the reported data are for the ITT population (76 patients). The median follow-up period was 13 months. The baseline patient characteristics and the baseline disease characteristics were evenly distributed across both the treatment groups in terms of the PS, histology and disease status (Table 1).
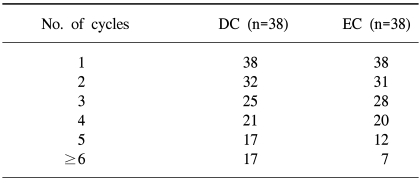
A total of 154 cycles of DC and 136 cycles of EC were administered, respectively. The median number of cycles was 4 (range: 1~9 cycles) in each arm (Table 2).
The relative dose intensity was 0.947 for docetaxel and 0.944 for etoposide. The percentage of cycles that was delayed was 17% for the DC and 14% for the EC. Leucopenia was the most common cause of cycle delay.
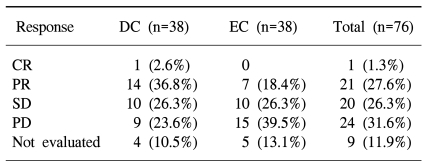
The tumor response rates in the ITT population are presented in Table 3. In the DC arm, 14 of the 38 patients (36.8%) had a PR and 1 (2.6%) had a CR. In the EC arm, 7 patients (18.4%) had a PR, but none had a CR. The ORR observed in the DC arm was significantly higher than that in the EC arm (p=0.023). The observed disease control rate was 65.7% and 44.7% of the patients who received DC and EC, respectively.
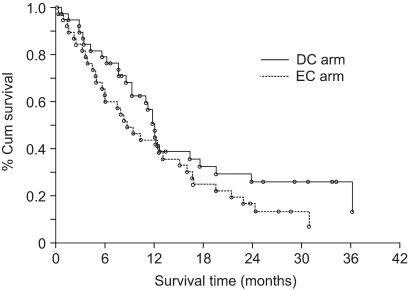
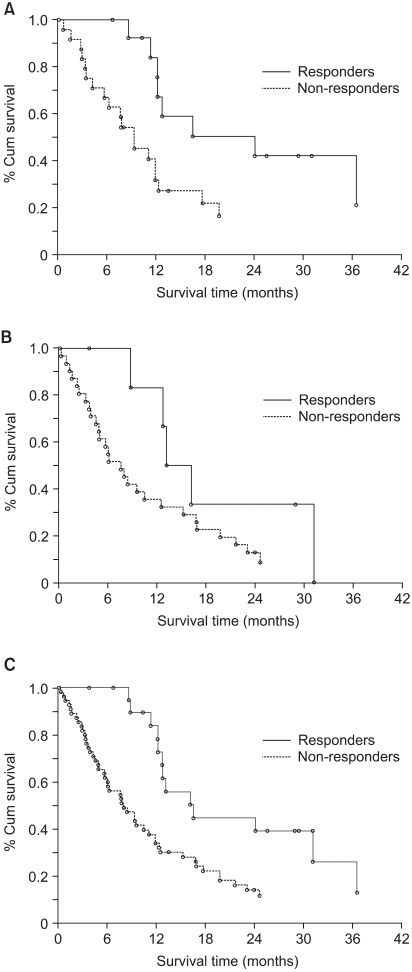
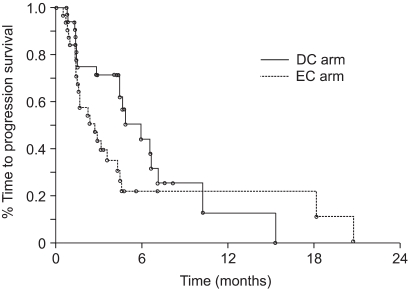
The median OS of the patients treated with DC and EC was 12.1 months (95% CI: 9.2~17.6) and 8.7 months (95% CI: 5.9 ~15.1), respectively. Fig. 1 shows the OS in both groups (p=0.168). The 1-year survival rates for the patients in both the groups were similar (50.4% for DC vs. 43.5% for EC). The observed 2-year survival rate in the DC arm (28.5%) was higher than that in the EC arm (16.3%), but the difference was not significant. The survival for patients with stage III disease, as compared with those with stage IV disease, was significantly different (p=0.0095). When patient survival was compared based on the tumor histology, the patients suffering with adenocarcinoma showed significantly longer survival than did those patients suffering with the other histological tumor types (p=0.0454). Other prognostic factors such as age, gender and PS did not affect patient survival. The responders to DC demonstrated significantly longer survival than did the non-responders (p=0.017). On the other hand, the response induced by EC did not affect overall survival (p=0.163) (Fig. 2). The median TTP was 5.9 months (95% CI: 4.4~7.1) in the DC arm and 2.7 months (95% CI: 1.5~4.3) in the EC arm (p=0.119) (Fig. 3).
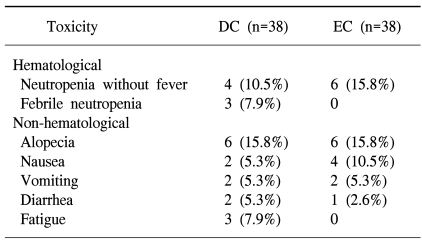
Seventy-six patients were evaluable for toxicity. Table 4 presents the patients who experienced treatment-related toxicities of NCI-CTC grade ≥3. Febrile neutropenia occurred in 3 patients in the DC arm, but this didn't occur in the EC arm. A higher incidence of neutropenia was recorded for the patients who received EC than for those patients who received DC; however, this difference was not statistically significant. The incidence of grades 3/4 non-hematological toxicities was similar in both arms, except for fatigue, which was predominant in the DC arm. No treatment-related deaths occurred in either of the treatment arms.
Go to : 
Different taxane combinations have been the mainstay of treatment for patients suffering with advanced NSCLC in many parts of the world. Two large phase III trials have been conducted to assess the efficacy of docetaxel-platinum combinations as compared to a vinca alkaloid or epipodophylotoxin with cisplatin (15,21). The TAX 326 study compared docetaxel and cisplatin (DC), or docetaxel and carboplatin (DCb), with vinorelbine and cisplatin (VC). That study demonstrated that clinically meaningful benefits were obtained with administering the two docetaxel plus platinum regimens (15). There was a strong trend for improved survival in the patients who received DC as compared with those patients who received VC. In addition, the patients in the docetaxel arms had a more favorable toxicity profile than did those patients in the VC arm. Perhaps most notable was the observation that the patients who had received either of the docetaxel regimens also experienced a consistent improvement in the QoL and also for the other measures of clinical benefit, including weight loss and PS. The response rate, TTP, median OS and 2-year survival rate for the patients who received DC were 31.6%, 22 weeks, 11.3 months and 21%, respectively. The DC combination proved to be better than the VC combination in terms of patient survival and the QoL (15). Additionally, a Japanese nationwide trial demonstrated that DC was superior to vindesine plus cisplatin in terms of both patient survival and the QoL (22).
While the DC has demonstrated superiority over the VC, a randomized phase III study of paclitaxel plus carboplatin (PCb) versus vinorelbine plus cisplatin (VC) for treating NSCLC revealed that PCb and VC had equivalent efficacy (23). Another phase III trial that compared EC with PCb for treating NSCLC showed an ORR of 14% for EC as compared to an ORR of 21.6% for PCb (p=0.059). Although this was not statistically significant, the paclitaxel combination appeared to have a better response rate than did the EC (24).
The Eastern Cooperative Group (ECOG) study 1594 examined 1,155 eligible and assessable patients who received combinations of DC, gemcitabine-cisplatin or PCb versus a paclitaxel-cisplatin (PC) control arm. None of the regimens that were compared showed significant improvement in the median, 1-year or 2-year survival. For the entire study population, the median survival was 7.9 months, and the 1-year and 2-year survival rates were 33% and 11%, respectively, for the study's two major arms. Grades 3/4 toxicity differences were observed between each comparator and the control arm. The DC combination was associated with more hypersensitivity reactions than was the PC combination (25).
The present trial was conducted to compare the efficacy and safety of DC and also the frequently used conventional EC regimen. The response rate was significantly higher in the DC group. Patients who were treated with DC showed a longer TTP and OS despite that there was no statistically significant difference. In particular, the responders who were treated with DC showed a significantly improved survival, while the responders who were treated with EC did not (p=0.017 and p=0.163, respectively). The difference in survival may not have been significant because of the relatively small sample size of our study.
One of the most important prognostic factors that this study examined was the histological sub-type, i.e., adenocarcinoma. In most studies, the histological types of NSCLC are not usually well described in terms of the chemotherapy response. The composition of the histological sub-types of NSCLC is not consistent across geographical regions or over time. In this trial, adenocarcinoma and squamous cell carcinoma were represented with almost the same frequency (48.8% in each arm), which is representative of the cancer patterns that are currently prevalent in Korea. It's rather interesting that patients with adenocarcinoma showed a significantly better survival (p=0.045). The majority of the patients enrolled in the two arms of a recent Japanese trial had adenocarcinoma (DC: 79.5%; EC: 68.2%) (21). It's yet to be determined if the adenocarcinoma sub-type is one of the factors to be considered when predicting the chemotherapy response and ultimately, the patients' survival.
Overall, the observed toxicities were mild and well tolerated by the patients in both treatment arms. Although the incidence of febrile neutropenia was higher in the DC arm, this malady was transient and it did not lead to sepsis or death. No treatment-related mortality was noted.
Go to : 
Although this trial did not achieve any significantly improved survival and TTP, the results confirmed the beneficial effects of combination therapy with using docetaxel and cisplatin compared with therapy using etoposide and cisplatin. The better response rates, the trend towards a longer median survival and a manageable safety profile suggest that the combination of docetaxel and cisplatin is an effective treatment option for locally advanced, recurrent or metastatic NSCLC.
Go to : 
References
1. Vokes EE, Bitran JD, Vogelzang NJ. Chemotherapy for non-small cell lung cancer. The continuing challenge. Chest. 1991; 99:1326–1328. PMID: 1645242.
2. De Vita VTJ, Hellman S, Rosenberg SA. Cancer: principles and practice of oncology. 2001. 6th ed. Philadelphia: J.B. Lippincott Co.;p. 925–983.
3. Bunn PA Jr, Kelly K. New chemotherapeutic agents prolong survival and improve quality of life in non-small cell lung cancer: a review of the literature and future directions. Clin Cancer Res. 1998; 4:1087–1100. PMID: 9607565.
4. Marino P, Pampallona S, Preatoni A, Cantoni A, Invernizzi F. Chemotherapy vs supportive care in advanced non-small-cell lung cancer. Results of a meta-analysis of the literature. Chest. 1994; 106:861–865. PMID: 7521815.
5. Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomized clinical trials. BMJ. 1995; 311:899–909. PMID: 7580546.
6. Grilli R, Oxman AD, Julian JA. Chemotherapy for advanced non-small-cell lung cancer: how much benefit is enough? J Clin Oncol. 1993; 11:1866–1872. PMID: 8410111.

7. Cullen MH, Billingham LJ, Woodroffe CM, Chetiyawardana AD, Gower NH, Joshi R, et al. Mitomycin, ifosfamide, and cisplatin in unresectable non-small-cell lung cancer: effects on survival and quality of life. J Clin Oncol. 1999; 17:3188–3194. PMID: 10506617.

9. Bunn PA Jr. The treatment of non-small cell lung cancer: current perspectives and controversies, future directions. Semin Oncol. 1994; 21(Suppl 6):49–59. PMID: 8052874.
10. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000; 18:2095–2103. PMID: 10811675.

11. Fossella FV, Lynch T, Shepherd FA. Second line chemotherapy for NSCLC: establishing a gold standard. Lung Cancer. 2002; 38(Suppl 4):5–12. PMID: 12480189.

12. Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004; 22:330–353. PMID: 14691125.

13. Zalcberg J, Millward M, Bishop J, McKeage M, Zimet A, Toner G, et al. Phase II study of docetaxel and cisplatin in advanced non-small-cell lung cancer. J Clin Oncol. 1998; 16:1948–1953. PMID: 9586914.

14. Georgoulias V, Androulakis N, Dimopoulos AM, Kouroussis C, kakolyris S, Papadakis M, et al. First-line treatment of advanced non-small-cell lung cancer with docetaxel and cisplatin: a multicenter phase II study. Ann Oncol. 1998; 9:331–334. PMID: 9602269.

15. Fossella F, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 Study Group. J Clin Oncol. 2003; 21:3016–3024. PMID: 12837811.

16. Klastersky J. Therapy with cisplatin and etoposide for non-small-cell lung cancer. Semin Oncol. 1986; 13(Suppl 3):104–114. PMID: 3020693.
17. Bunn PA Jr. The expanding role of cisplatin in the treatment of non-small-cell lung cancer. Semin Oncol. 1989; 16(Suppl 6):10–21. PMID: 2548280.
18. Chung HG, Choi JH, Chung YS, Kim DL, Lee YS, Chang J, et al. Phase II trial of sequential VP-16, cisplatin combination chemotherapy and radiotherapy for locally advanced (stage III) non-small cell lung cancer. J Korean Cancer Assoc. 1991; 23:131–139.
19. Kim KH, Oh SY, Jeong HS, Lee JT, Kim WS, Kim HJ, et al. Prolonged oral etoposide in combination with intravenous cisplatin for advanced non-small cell lung cancer. J Korean Cancer Assoc. 1999; 31:105–111.
20. Paek CW, Yoon SY, Seo JH, Choi CW, Kim BS, Shin SW, et al. Concurrent chemoradiation therapy with cisplatin and oral etoposide for locally advanced non-small cell lung cancer. J Korean Cancer Assoc. 2000; 32:682–689.
21. Akehurst RL, Beinert T, Crawford J, Crino L, Debus J, Edcersberger F, et al. Consensus on medical treatment of non-small cell lung cancer. Lung Cancer. 2002; 38(Suppl 3):S3–S13.

22. Kubota K, Watanabe K, Kunitoh H, Noda K, Ichinose Y, Katakami N, et al. Phase III randomized trial of docetaxel plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small-cell lung cancer: the Japanese Taxotere Lung Cancer Study Group. J Clin Oncol. 2004; 22:254–261. PMID: 14722033.

23. Kelly K, Crowley J, Bunn PA Jr, Presant CA, Grevstad PK, Moinpour CM, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non--small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001; 19:3210–3218. PMID: 11432888.

24. Belani CP, Lee JS, Socinski MA, Robert F, Waterhouse D, Rowland K, et al. Randomized phase III trial comparing cis platin-etoposide to carboplatin-paclitaxel in advanced or metastatic non-small cell lung cancer. Ann Oncol. 2005; 16:1069–1075. PMID: 15860487.
25. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–98. PMID: 11784875.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download