Abstract
Purpose
The 10-year overall survival rate following conventional treatments for patients with limited-stage Hodgkin's disease (HD) exceeds 90%. However, the clinical features and treatment outcome of HD in Korea have not been extensively characterized due to its low incidence. In this study, we attempted to analyze the treatment outcome of different modalities in limited stage HD patients.
Materials and Methods
Twenty one Hodgkin's disease patients, referred to the Samsung Medical Center between January 1997 and December 2003, were enrolled in this study. Limited stage Hodgkin's disease was subdivided into low and high risk groups. All evaluable patients received treatment.
Results
There were 13 and 8 patients in the low and high risk groups, respectively. Eighteen patients (86%) obtained complete response (CR) and 3 patients (14%) achieved an undetermined complete response (CRu). Fourteen (67%), 4 (19%) and 3 (14%) cases received combination chemotherapy, radiotherapy alone and chemotherapy alone, respectively. Four cases relapsed and 2 obtained a second CR. The 5-year overall and disease-free survival rates were 90 and 72%, respectively, for all patients. The median follow-up duration was 31 months. There was no difference in disease free survival (DFS) between the low and high risk groups. Although 12 cases had neutropenia greater than grade III, none experienced neutropenic fever.
With improvements in chemotherapy and radiation therapy, Hodgkin's disease, a representative malignant tumor, has changed from a fatal to a curable disease (1). Kaplan (2) first introduced radiation therapy in 1950s, which has subsequently played a major role in the treatment of Hodgkin's disease.
In 1964, Devita (3) introduced the combination chemotherapy of mechlorethamine, vincristine, procarbazine and prednisone (MOPP), which drastically changed the treatment of Hodgkin's disease. However, MOPP and its derivatives had the limitation of only 50% curability, with the complications of sterility and acute leukemia. Various chemotherapy regimens for Hodgkin's disease have been attempted to overcome these drawbacks, and the combination regimen of Adriamycin, Belomycin, Vinblastine and Dacarbazine (ABVD) has been established as the standard chemotherapy for Hodgkin's disease (6).
For the convenience of its treatment and the analysis of clinical studies, Hodgkin's disease is classified into the limited and advanced stages. The limited stage is equivalent to stages 1 and 2 of the Ann Arbor classification, which can be subclassified into the high risk with B symptoms or a bulky disease and the low risk, early stage, groups. Early Hodgkin's disease has a long-term survival rate of over 90%, so recent treatments have been focused on the reduction of long-term complications. Studies showing comparable effectiveness and reduced complications of treatments have recently been performed by combining radiation therapy and chemotherapy. In non-Hodgkin's disease, chemoradiotherapy has been reported to have a similar superior therapeutic effect, but a reduced toxicity over that of chemotherapy alone (7). We performed this study to examine the therapeutic outcome and complications of limited stage of Hodgkin's disease experienced at the Samsung Medical Center.
The study was performed on 21 patients, histologically diagnosed with clinical stage I and II adult Hodgkin's diseases at the Samsung Medical Center, between January 1997 and December 2003, and their data retrospectively analyzed. The clinical stage of all patients was classified using the Cotswold criteria (8). The staging was strictly clinical, including medical history and physical examination, complete blood counts, peripheral blood smear, erythrocyte sedimentation rate (ESR), renal and liver functions, chest x-ray examination, biopsies of the invaded lymph nodes, chest and abdominal computed tomography (CT) and bone marrow biopsy. No open abdominal surgery was performed to determine the disease stage.
The limited stage was analyzed by classifying the low and high risk groups. The definition of a bulky disease was a mediastinal mass occupying more than a third of the thoracic diameter and/or a nodal disease greater than 10 cm.
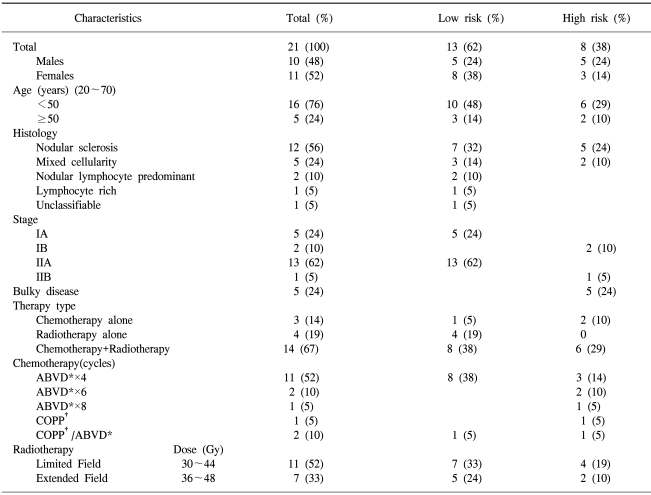
Among a total of 78 patients diagnosed between July 1994 and December 2003, 21 (26.9%) cases were stage I and II diseases. The main characteristics of the patients are shown in Table 1. There were 10 (48%) male and 11 (52%) female cases, with a median age of 31 years, ranging from 20 to 70). Histologically, 12 (57%), 5 (24%), 2 (10%) and 2 (10%) cases were nodular sclerosis, mixed cellularity, nodular lymphocyte-predominant and other types, respectively. The disease stages were evaluated as I in 7 (33%) and II in 14 (67%) cases. Five (24%) cases had a bulky mass and three (14%) had B symptoms. 14 (67%), 4 (19%) and 3 (14%) cases were treated by chemoradiotherapy radiation therapy only and chemotherapy alone, respectively. There were 13 (62%) and 8 (38%) cases in the low and high risk groups, respectively.
The chemotherapy regimens employed were ABVD, C-MOPP and alternating C-MOPP/ABVD, with each administered for at least 4 cycles. The radiation therapy was started after the last cycle of chemotherapy. The total doses ranged from 30 to 48 Gy, delivered to either the involved or extended field.
The physical examination, routine laboratory tests and chest x-ray were repeated in all patients after every cycle of chemotherapy. The treatment response was assessed clinically and by repeating all chest and abdominal CT on those that were positive at diagnosis. These tests were performed within 4 weeks of ABVD chemotherapy completion. Follow-ups were performed every 3 months for the first 2 years, and every 6 months thereafter.
The effectiveness was scored as 1 of 5 grades, according to the NCI criteria, these being: complete response (CR), complete response unconfirmed (CRu), partial response (PR), stable disease (SD) and progressive disease (PD).
The treatment toxicity was evaluated by general blood and chemistry tests prior to treatment, simple chest radiography and a systemic review.
The overall survival time was from the day of diagnosis to death or the last day of follow up and the disease-free survival was from the day a complete response was detected to the day of the confirmed recurrence, death or last day of follow up. The patterns of disease free and overall survivals were estimated using the Kaplan-Meier method, and for the analysis of prognostic factors, a log-rank test was performed.
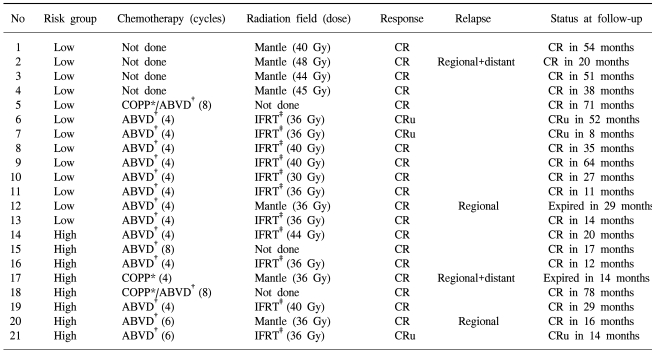
18 (86%) cases achieved CR and 3 (14%) CRu. All cases responded to the treatments. There were 11 (85%) cases of CR and 2 (15%) cases of CRu in 13 cases of low risk group. In the high risk group, CR was 7 (87%) cases and CRu was 1 (13%) case (Table 2).
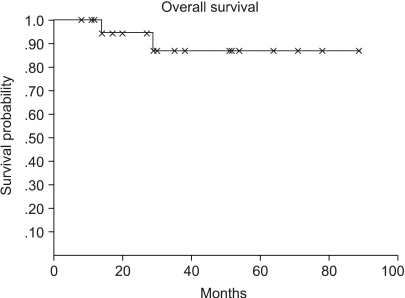
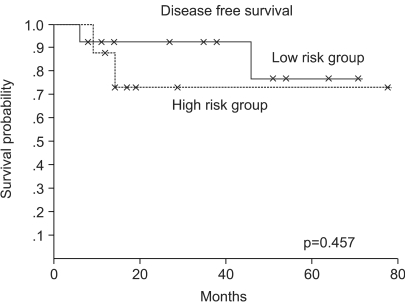
The 5-year overall and disease free survival rates were 90 and 73%, respectively, and when classified according the low and high risk groups, were 92 and 77% and 88 and 73%, respectively (Fig. 1, 2). The median follow up period was 31 months. Four (19%) cases relapsed and 2 achieved CR by salvage chemotherapy and have been followed without evidence of a relapse, and 2 cases died. One case died of infection during salvage chemotherapy, and the other progressed and died, even after stem cell transplantation.
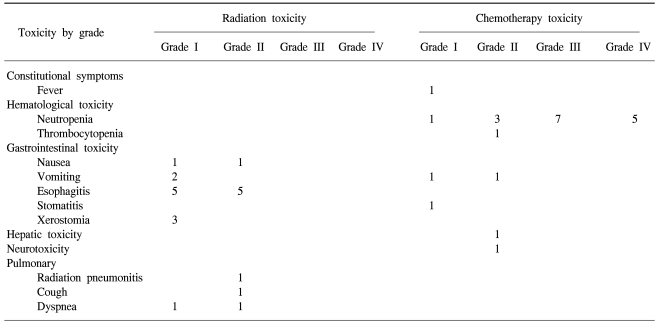
Evaluation of the radiation therapy toxicity was possible in 15 of the 18 cases. 12 (80%) cases developed esophagitis, but with severities lower than grade II. Grade I and II nausea were detected in 2 cases. The other radiation therapy toxicities included xerostomia in 3, dyspnea in 2 and radiation pneumonia in 1 case. Evaluation of the chemotherapy toxicity was possible in 17 cases, and occurred in 16 cases. Neutropenia was detected in 16 (94%) cases, with 12 (71%) being grade III or higher; however, no infection developed due to neutropenia. Hepatotoxicity occurred in 2 cases and thrombocytopenia in 1. A fever caused by bleomycin was detected in 1 case (Table 3).
The incidence of Hodgkin's disease in Korea has been reported to be rare. In a study on lymphomas by Lee (9), the ratio of non-Hodgkin's lymphomas and Hodgkin's disease was 5.7:1, and Kim (10) reported a 17% incidence of Hodgkin's disease in malignant lymphomas.
The therapy for limited stage Hodgkin's disease has changed drastically over the last 20 years. After the introduction of radiation therapy for Hodgkin's disease, in 1950s, the standard treatment modalities for limited stage Hodgkin's disease were a staging laparotomy and splenectomy, followed by wide field irradiation. Park described an 87% 5-year overall survival rate in early Hodgkin's disease (11). Although the reported long term disease-free survival rates were favorable, secondary complications induced by radiation therapy and recurrence in the high risk group were noted. As a consequence, the necessity for chemotherapy or chemoradiotherapy has been proposed (12).
Devita et al (3). reported a complete remission rate of 81%, with a median survival time longer than 42 months in advanced Hodgkin's patients treated with the MOPP combination chemotherapy. This suggested the curative potential of chemotherapy for patients with Hodgkin's disease. However, due to the side effect the MOPP regimen, due to mechlorethamine and vincristine, therapies reducing the side effects and increasing the efficacy have been developed. The Milan group proposed the ABVD regimen (6), which has partial non-cross resistance with MOPP and can cure about 20% of patients not cured using the aforementioned regimen. ABVD also has less pronounced longterm toxicity, very infrequently causes sterility or premature menopause and is less leukemogenic. In limited stage Hodgkin's disease treated with ABVD chemotherapy alone, the outcomes were comparable to those of radiation therapy (13).
Chemoradiotherapy, which combines chemotherapy and radiation therapy, has been reported to lower the recurrence rate compared with radiation or chemotherapy alone (14~17). After the introduction of chemoradiotherapy, the treatment for Hodgkin's disease focused on reduction of the toxicity, while maintaining the efficacy. It has been reported that there is no difference between involved field and wide area irradiation in a large number of phase III clinical studies on limited stage Hodgkin's disease (18,19). By administering chemoradiotherapy, it was possible, not only to reduce the irradiation area, but also the irradiation dose. Diehl (20) reported a difference when the effectiveness of 20 Gy and 30 Gy were compared in low risk Hodgkin's disease. In our study, irradiation doses from 36 to 48 Gy, with either involved or an extensive irradiation fields, were used. No differences in the relapse and mortality rates were found in relation to the radiation dose. The combination regimen allowed the chemotherapy as well as the radiation doses to be reduced. The same theoretical considerations applied to the irradiation dose are also relevant when considering reduction of the chemotherapy dose used in combined modality treatments. Diehl reported no difference with between 2 and 4 cycles of ABVD chemotherapy when combined with radiotherapy (20). However, generally patients with limited stage Hodgkin's disease in the high risk group require more frequent or a higher dose of chemotherapy and radiation therapy. Although this study was limited to a small sized population and a retrospective method, the treatment outcomes were favorable. No differences in the survival or disease-free survival rates were detected in the analyses of the patients with a limited stage disease after their classification into the low risk and high risk groups. In a comparison of radiation therapy alone, chemotherapy alone and chemoradiotherapy groups no statistically significant results were observed, but the significance of this study may be limited due to the small study population of 4, 3 and 14 cases, respectively.
The acute complications due to radiation therapy for Hodgkin's disease are usually manageable by symptomatic therapies. Twelve (80%) of the 15 cases treated with radiation developed esophagitis in this study, but all patients had lower than grade II toxicity. Therefore, no termination or delay due to complications occurred. With regard to the side effects of chemotherapy, higher than grade III neutropenia developed in 70%, but no fever or infection due to neutropenia was detected.
References
1. Rosenberg SA, Kaplan HS. The evolution and summary results of the Stanford randomized clinical trials of the management of Hodgkin's disease: 1962-1984. Int J Radiat Oncol Biol Phys. 1985; 11:5–22. PMID: 3881376.

2. Kaplan HS. The radical radiotherapy of regionally localized Hodgkin's disease. Radiology. 1962; 78:553–561. PMID: 14453744.

3. Devita VT Jr, Serpick AA, Carbone PP. Combination chemotherapy in the treatment of advanced Hodgkin's disease. Ann Intern Med. 1970; 73:881–895. PMID: 5525541.
4. Harding MJ, McNulty LJ, Paul J, Lee F, Brown A, Soukop M. Mechlorethamine, vinblastine, procarbazine and prednisolone (MVPP) for advanced Hodgkin's disease. Eur J Cancer. 1991; 27:1002–1006. PMID: 1832882.

5. Hancock BW, Vaughan Hudson G, Vaughan Hudson B, Haybittle JL, Bennett MH, MacLennan KA, et al. British national lymphoma investigation randomised study of MOPP (mustine, Oncovin, procarbazine, prednisolone) against LOPP (Leukeran substituted for mustine) in advanced Hodgkin's disease--long term results. Br J Cancer. 1991; 63:579–582. PMID: 2021542.
6. Bonadonna G, Zucali R, Monfardini S, De Lena M, Uslenghi C. Combination chemotherapy of Hodgkin's disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer. 1975; 36:252–259. PMID: 54209.

7. Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, Grogan TM, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and highgrade non-Hodgkin's lymphoma. N Engl J Med. 1998; 339:21–26. PMID: 9647875.

8. Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989; 7:1630–1636. PMID: 2809679.

9. Lee JM, Hwang KS, Choi GW, Kang DY. Malignant lymphoma in Korea. Korean J Hematol. 1977; 12:1–20.
10. Kim KW, Chang YB, Kim MC, Son CH. Clinical observation on 55 cases of Hodgkin's disease. Korean J Hematol. 1982; 17:35–44.
11. Park YH, Suh CO, Kim GE, Loh JK. Radiotherapy in Hodgkin's disease. J Korean Cancer Assoc. 1992; 24:277–287.
12. Connors JM, Noordijk EM, Horning SJ. Hodgkin's lymphoma: basing the treatment on the evidence. Hematology (Am Soc Hematol Educ Program). 2001; 178–193. PMID: 11722984.

13. Cimino G, Biti GP, Anselmo AP, Maurizi Enrici R, Bellesi GP, Bosi A, et al. MOPP chemotherapy versus extended-field radiotherapy in the management of pathological stages I-IIA Hodgkin's disease. J Clin Oncol. 1989; 7:732–737. PMID: 2715803.

14. Sieber M, Franklin J, Tesch H. Two cycles ABVD plus extended field radiotherapy is superior to radiotherapy alone in early stage Hodgkin's disease:Results of the German Hodgkin's Lymphoma Study Group(GHSG) Trial HD7. Blood. 2002; 100:A341.
15. Press OW, LeBlanc M, Lichter AS, Grogan TM, Unger JM, Wasserman TH, et al. Phase III randomized intergroup trial of subtotal lymphoid irradiation versus doxorubicin, vinblastine, and subtotal lymphoid irradiation for stage IA to IIA Hodgkin's disease. J Clin Oncol. 2001; 19:4238–4244. PMID: 11709567.

16. Laskar S, Gupta T, Vimal S, Muckaden MA, Saikia TK, Pai SK, et al. Consolidation radiation after complete remission in Hodgkin's disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy: is there a need? J Clin Oncol. 2004; 22:62–68. PMID: 14657226.

17. Bonfante V, Viviani S, Devizz I. 10-year experience with AVBD plus radiotherapy; subtotal nodal (STNI) versus involved-field (IFRT) in early-stage Hodgkin's disease. Proc Am Soc Clin Oncol. 2001; 20:281a.
18. Engert A, Schiller P, Josting A, Herrmann R, Koch P, Sieber M, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin's lymphoma: results of the HD8 trial of the German Hodgkin's Lymphoma Study Group. J Clin Oncol. 2003; 21:3601–3608. PMID: 12913100.

19. Ferme C, Eghbali H, Hagenbeek A, Brice P, Meder J, Carde P, et al. MOPPABV hybrid and irradiation in unfavorable supradiaphragmatic clinical stages I-II Hodgkins disease: Comparison of three treatment modalities. Preliminary results of the EORTC-GELA H8-U randomized trial in 995 patients. Blood. 2000; 96:A576.
20. Diehl V, Stein H, Hummel M, Zollinger R, Connors JM. Hodgkin's lymphoma: biology and treatment strategies for primary, refractory, and relapsed disease. Hematology (Am Soc Hematol Educ Program). 2003; 225–247. PMID: 14633784.

21. Multani PS, Grossbard ML. Staging laparotomy in the management of Hodgkin's disease: Is It Still Necessary? Oncologist. 1996; 1:41–55. PMID: 10387968.

22. Mauch PM, Kalish LA, Marcus KC, Coleman CN, Shulman LN, Krill E, et al. Second malignancies after treatment for laparotomy staged IA-IIIB Hodgkin's disease: long-term analysis of risk factors and outcome. Blood. 1996; 87:3625–3632. PMID: 8611686.

23. van Leeuwen FE, Klokman WJ, Hagenbeek A, Noyon R, van den Belt-Dusebout AW, et al. Second cancer risk following Hodgkins disease: a 20-year follow-up study. J Clin Oncol. 1994; 12:312–325. PMID: 8113838.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download