This article has been retracted. See "Retraction: Tetraarsenic Oxide-mediated Apoptosis in a Cervical Cancer Cell Line, SiHa" in Volume 39 on page 48.
Abstract
Purpose
Diarsenic oxide, As2O3, has been reported to be effective in treating acute leukemia, and induce apoptosis in many tumor cells. In this study, the ability of a novel arsenical compound, As4O6 (tetraarsenic oxide), along with As2O3, for its ability to induce cell growth inhibition, as well as apoptosis, in human cervical cancer cells, SiHa cells, were evaluated in vitro.
Materials and Methods
To examine the levels of apoptosis, SiHa cells were given two sensitive doses, 0.5 and 1 µM, of arsenical compounds, and a DNA fragmentation assay and FACS analysis were then conducted. In addition, a Western blotting assay was performed to identify target molecules for apoptosis.
Results
Both As2O3 and As4O6 induced dosedependent inhibition of SiHa cell proliferation. In particular, As4O6 was more effective at suppressing SiHa cell growth than As2O3. In parallel with the inhibition of cell proliferation, As4O6 caused a significantly greater increase in the sub-G1 cell population than As2O3, as determined by propidium iodide DNA staining. This was confirmed by a DNA fragmentation assay and annexin V staining. The Western blotting analysis also showed that the expression of proliferating cell nuclear antigen (PCNA) was suppressed to a significantly greater extent by As4O6 than As2O3 at a dose of 0.5 µM. However, the apoptosis-related protein, Bax, was expressed to a significantly greater extent due to As4O6 than As2O3.
Arsenical compounds are distributed as a natural toxicant, with no color, taste or smell. Diarsenic trioxide, As2O3, has been reported to induce almost complete remission of acute promyelocytic leukemia (APL) (1~3). Cytopathological studies have also shown that As2O3 induces apoptosis in APL cells. Recent reports have shown that As2O3 down-regulates the expression of the bcl-2 gene and induces the expression of the apoptosis-related proteins, caspases, as well as the degradation of promyelocytic leukemia (PML) and promyelocytic leukemia gene/retinoic acid receptor (PML/RAR) α proteins in APL cells (2,4). Similarly, arsenic trioxide has been shown to suppress the growth of tumor cells by cell cycle arrest, induction of cyclin-dependent kinase (CDK) inhibitors and apoptosis in a myeloma cell line, MC/CAR (5). Arsenic trioxide also causes cell death through apoptosis in a human leukemia cell line, NB4 (6), human papillomavirus (HPV) 16 infected cervical carcinoma cells (7) and human pancreatic cancer cells (8,9), which collective suggest that arsenic trioxide has anti-tumor activity in a variety of tumor cells.
Cervical cancer is mostly caused by infection within the high risk HPV group (10~12). After a high risk HPV infection, two viral oncogenic proteins, E6 and E7, play a critical role in the induction of cervical cancers by their interaction with p53 and retinoblastoma protein (pRb), respectively, for the inactivation of these cellular regulatory proteins (13~15). Presently, surgical, radiation and chemo- therapies have been used as approaches, but with limited success. However, the early detection of cervical cancer using the Pap smear has contributed to reducing the incidence of cervical cancers. However, relapsing cervical cancers have been problematic, adding importance to the development of anti-cervical cancer drugs.
Here, the ability of a new arsenical compound, As4O6 (tetraarsenic oxide), along with As2O3, were evaluated for their ability to suppress cell growth in human cervical carcinoma cells, SiHa cells. As4O6 was observed to be more effective at inhibiting SiHa cell growth than As2O3. Furthermore, As4O6 caused significantly greater increases in the sub-G1 cell population, DNA ladder formation and annexin V staining than As2O3. Furthermore, Western blotting analysis showed the expression of a cell proliferation marker was more significantly suppressed by As4O6 than As2O3. However, the expression of apoptosis-related protein was significantly increased by As4O6 than As2O3. Thus, these data suggest that a novel arsenic compound, As4O6, possesses more potent anti-proliferative effects on human cervical cancer cells, also with the induction of apoptosis, in vitro, compared to As2O3.
HPV 16, immortalized a human cervical carcinoma cell lines, SiHa cells, containing wild type p53, were incubated at 37℃ in DMEM supplemented with 5% fetal bovine serum, 0.37% sodium bicarbonate, 30 µM HEPES and 100 µg/ml streptomycin/penicillin (cDMEM), in a CO2 incubator.
The As2O3 was purchased from Sigma and the As4O6 (16~19) was provided from Chonjisan, Co., Seoul, Korea. The chemical structures of both As2O3 and As4O6 are shown in Fig. 1. These chemicals were diluted in phosphate-buffered saline (PBS), to a final concentration of 10-3 M, and kept at 4℃. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma, which was dissolved in PBS to a final concentration of 5 mg/ml.
As a cell growth inhibition assay, the MTT assay was performed. First, SiHa cells (1×103 cells/well) were divided into 96 well plates in 100 µl of cDMEM. After 24 h incubation, the cells were treated with different amounts of the two arsenic compounds. After 4 days incubation, 20 µl of MTT solution was added to each well of the plates, which were further incubated for 4 h at 37℃. The cell media were replaced with 100 µl of dimethyl sulfoxide (Sigma) per well. The plate was shaken for 10 sec and the optical density (OD) then measured at 570 nm, using an enzyme-linked immunosorbent assay-Reader (Spectromax 250, Molecular Devices). The growth inhibition rate (%) was calculated as follows: OD of non-treatment-OD of drug treatment/OD of non-treatment×100.
SiHa cells were divided into 5×105 cells per 100 mm dish plate. After 24 h incubation, different amounts of arsenic compounds were added to the cells, which were further incubated for 48 h at 37℃. The cells were then centrifuged, with lysis buffer [0.8% SDS, 0.1 M NaCl, 0.1 M EDTA, 50 µM Tris-HCl (pH8.0)] added to the subsequent cell pellets, followed by the addition of 20 µg/ml proteinase K (Sigma) and further incubated for 4 h at 56℃. DNA was extracted by phenol/chloroform treatment. Five mg of extracted DNA was analyzed on a 2% agarose gel containing ethidium bromide (0.1 µg/ml). The DNA ladder formation was visualized under UV light.
Cells were washed twice with PBS and then resuspended in 1×binding buffer (10 µM Hepes/NaOH, pH 7.4, 140 µM NaCl, 2.5 µM CaCl2). 5 µl of Annexin V-FITC and 10 ml of propidium iodide (BD, San Jose, CA) were added to 1×105 cells per tube, followed by incubation at 22℃ for 15 min. 100 µl of 1×binding buffer was added to each tube, and the cells then analyzed by flow cytometry (BD). To analyze for the DNA contents, ethanol-fixed cells were incubated with RNase A (10 mg/ml) and propidium iodide (400 µg/ml), and then shaken for 1 h at 37℃ in the dark. The samples were read using flow cytometry (BD). The cell debris and fixation artifacts were gated out, and the G0/G1, S and G2/M populations were quantified using the CellQuest program.
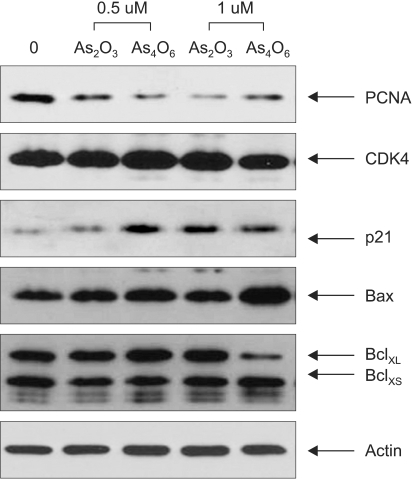
SiHa Cells were treated with 0.5 and 1 µM of As2O3 and As4O6 for 48 h. The cell lysates (approximately 30 µg of protein) was separated on 12% polyacrylamide SDS-gels and transferred to the nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). The membrane was then immersed in blocking buffer (5% skimmed milk and 0.1% Tween 20 in PBS, pH 7.4) for 1 h, at room temperature, and then incubated overnight with the primary antibodies (SantaCruz Biotechnology, Inc., Santa Cruz, CA), PCNA (1:200), CDK4 (1:200), p21 (1:200), Bax (1:200), Bcl-XL/Bcl-XS (1:500) and actin (1:5000), prepared in blocking buffer at 4℃. After incubation, the membrane was probed with the horseradish peroxidase-labeled anti-mouse IgG antibody (1:5000), prepared in PBS (containing of 0.05% Tween 20 and 5% skim milk powder), for 30 min at room temperature. The proteins bands in the membrane were detected using an enhanced chemiluminescence detection system (Amersham, Buckinghamshire, UK), and visualized using autoradiography with an X-ray film (Amersham).
To investigate whether a new arsenical compound, As4O6, could suppress the growth of human cervical cancer cells, SiHa cells were treated with increasing amounts (0.5~5 µM) of As4O6 for 48 h. As a positive control, cells were also treated with As2O3. As shown in Fig. 2, the growth of SiHa cells was unaffected by their treatment with 0.5 µM As2O3. However, 1 µM As2O3 showed approximately 50% cell growth inhibition, while As2O3, ranging from 2 to 5 µM, displayed complete growth inhibition of the cancer cells. With As4O6, 0.5 µM displayed approximately 50% growth inhibition of the cancer cells, but within the range 1 to 5 µM showed complete growth inhibition of the cancer cells. This illustrates that As4O6 is more effective than As2O3 at inhibiting the growth of human cervical cancer cells in vitro.
Next, the levels of apoptosis due to the addition of the two most sensitive doses, 0.5 and 1 µM, of the arsenical compounds (As2O3 and As4O6) were examined. As shown in Fig. 3A, As4O6 induced DNA ladder formation at 0.5 µM, indicative of apoptosis. However, few DNA ladders were formed on the addition of 0.5 µM As2O3. This suggests that As4O6 is more effective for the induction of apoptosis compared to As2O3. This pattern of apoptosis was confirmed by flow cytometry (Fig. 3B). In particular, As4O6 displayed 25% sub-G1 cell populations at 0.5 µM. However, few sub-G1 cell populations were observed on the addition of 0.5 µM As2O3. Similarly, 1 µM of each As2O3 and As4O6 showed 30 and 50% sub-G1 cell populations, respectively. Therefore, this data confirms that As4O6 is more potent at inducing apoptosis in SiHa cells.
Next, the different apoptotic cell populations induced by these two drugs were counted. As shown in Fig. 4, the propidium iodide (+)/Annexin V (+) double positive cell populations (late apoptotic group) were 3.7, 3.7 and 4.8% at 0.0, 0.5 and 1 µM As2O3, respectively. The propidium iodide (-)/Annexin V (+) cell populations (early apoptotic group) were 1.4, 1.9 and 4.4% at 0.0, 0.5 and 1 µM As2O3, respectively. However, the propidium iodide (+)/Annexin V (+) double positive cell populations were 3.7, 8.0 and 11.5% at 0.0, 0.5 and 1 µM As4O6, respectively. Similarly, the propidium iodide (-)/Annexin V (+) cell populations were 1.4, 2.5 and 8.1% at 0.0, 0.5 and 1 µM As4O6, respectively. This shows that As4O6 induces greater increases in early and late apoptotic cells compared to As2O3.
To compare the anti-growth effects induced by As2O3 and As4O6 at the cell proliferation- and apoptosis-related protein levels, the cells were treated with the two arsenical compounds at 0.5 and 1 µM. As shown in Fig. 5, the expression of the cell proliferation marker, PCNA, was decreased by both arsenic compounds, whereas those of the apoptosis-related proteins, Bax and p21, were conversely increased. In particular, As4O6 was found to significantly inhibit the expressions of PCNA and Bcl-XL compared to As2O3 at 0.5 µM and 1 µM, respectively. Similarly, the expressions of Bax and p21 were significantly increased by As4O6 compared to As2O3 at 1 µM and 0.5 µM, respectively. However, the expressions of CDK4, Bcl-XS and actin were unaffected by the two arsenic compounds. This illustrates that As4O6 can induce apoptosis, at least via the activation of the apoptosis-related protein, Bax, to a significantly greater level compared to As2O3.
The anti-tumor functions of the arsenic compound, As2O3, have been reported in leukemia both in vivo and in vitro (1~4). Here, As2O3 was also observed to induce apoptosis and cell growth inhibition in SiHa cell lines. This supports the previous findings where arsenic trioxide was found to induces anti-tumor effects via the induction of tumor cell apoptosis (1~5, 8, 9). In the case of promyeloleukemic cells, As2O3 has been found to down-regulate the expressions of bcl-2 and PML/RARα/PML proteins, which correlated with apoptosis (2,4). As2O3 also induces apoptosis in human pancreatic cancer cells, via changes in the cell cycle and caspase activation (8,9). Dai et al. also reported that the glutathione redox system was associated with apoptosis due to As2O3 (20). In one article, As2O3 was reported to inhibit cell growth and induce apoptosis in different cancers, including leukemia, myeloma, lymphoma and other solid tumors (21), suggesting apoptosis is a likely mechanism of arsenic trioxide in the suppression of tumor cell growth both in vivo and in vitro.
Herein, As4O6 was observed to be more effective at suppressing SiHa cells proliferation in vitro compared to As2O3. In particular, As4O6 showed significant anti-growth activity (approximately 50% cell growth inhibition) at a dose of 0.5 µM, but As2O3, at this concentration displayed few anti-growth effects. However, 1 µM As2O3 showed approximately 50% growth inhibition, which was consistent with our observation, where at a dose of 0.5 µM both DNA fragments and a sub-G1 cell population were significantly generated by As4O6 compared to As2O3. This is in line with the greater induction of early and late apoptotic cells observed in this study due to As4O6. These data support the notion that As4O6 could be a more potent drug against cervical cancer cell growth via apoptosis. The dose effects of arsenic trioxide on growth inhibition were consistent with many previous reports (5,7). The expression of the cell proliferation marker, PCNA (22), was also observed to be decreased by the two arsenic compounds. In particular, As4O6 significantly inhibited the expression of PCNA expression compared to As2O3. This is in line with our observation where As4O6 was shown to significantly inhibit the growth of cancer cells compared to As2O3. Furthermore, the expressions of the apoptosis-related proteins, Bax (23) and p21 (24), were increased by both arsenic compounds. In particular, the expression of Bax was significantly increased by As4O6 compared to As2O3. This correlates well with our observation that As4O6 significantly induced apoptosis in SiHa cells compared to As2O3, which is also compatible with the previous finding where As2O3 increased the expressions of the p21 and Bax proteins in human pancreatic cancer cells (9). However, the expressions of CDK4, Bcl-XS and actin were unaffected by the two arsenic compounds. Thus, these data suggest that As4O6 mediates apoptosis, at least via the activation of the Bax pathway, in human cervical cancer cells, SiHa cells. Conversely, our thinking was that the BclXL (25) and p21 proteins were more sensitive to changes in the concentrations of arsenic compounds.
The data presented in this study suggest that tetraarsenic oxide, As4O6, is more effective at suppressing the growth of SiHa cell growth compared to As2O3. In parallel with the inhibition of cell proliferation, As4O6 caused significant increases in the sub-G1 cell population, DNA ladder formation and annexin V staining compared to As2O3. Furthermore, the Western blotting analysis showed the expression of the apoptosis-related protein, Bax, was significantly increased by As4O6 compared to As2O3. Taken together, these findings suggest that a novel arsenic compound, As4O6, possesses more potent anti-growth effects toward human cervical cancer cells, with the induction of apoptosis in vitro, which might provide a new drug choice in the treatment of HPV-associated cervical cancer.
References
1. Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997; 89:3354–3360. PMID: 9129042.
2. Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti L, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998; 339:1341–1348. PMID: 9801394.

3. Zhang P, Wang SY, Hu LH, Shi FD, Qiu FD, Hong RJ, et al. Arsenic trioxide treated 72 cases of acute promyelocytic leukemia. Chi J Hematol. 1996; 17:58–70.
4. Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-PARalpha/PML proteins. Blood. 1996; 88:1052–1061. PMID: 8704214.
5. Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, et al. Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res. 2000; 60:3065–3071. PMID: 10850458.
6. Gurr JR, Bau DT, Liu F, Lynn S, Jan KY. Dithiothreitol enhances arsenic trioxide-induced apoptosis in NB4 cells. Mol Pharmacol. 1999; 56:102–109. PMID: 10385689.

7. Zheng J, Deng YP, Lin C, Fu M, Xiao PG, Wu M. Arsenic trioxide induces apoptosis of HPV16 DNA- immortalized human cervical epithelial cells and selectively inhibits viral gene expression. Int J Cancer. 1999; 82:286–292. PMID: 10389765.
8. Li X, Ding X, Adrian TE. Arsenic trioxide inhibits proliferation and induces apoptosis in pancreatic cancer cells. Anticancer Res. 2002; 22:2205–2213. PMID: 12174905.
9. Li X, Ding X, Adrian TE. Arsenic trioxide induces apoptosis in pancreatic cancer cells via changes in cell cycle, caspase activation and GADD expression. Pancreas. 2003; 27:174–179. PMID: 12883267.

10. Cullen AP, Reid R, Campion M, Lorincz AT. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol. 1991; 65:606–612. PMID: 1846186.

11. Lorincz AT, Temple GF, Kurman RJ, Jenson AB, Lancaster WD. Oncogenic association of specific papillomavirus types with cervical neoplasia. J Natl Cancer Inst. 1987; 79:671–677. PMID: 2821311.
12. zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989; 49:4677–4681. PMID: 2547512.
13. Scheffner M, Werness BA, Heibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990; 63:1129–1136. PMID: 2175676.

14. Scheffner M, Munger K, Bryne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci USA. 1991; 88:5523–5527. PMID: 1648218.

15. Werness BA, Levine AJ, Howley PM. Association of HPV type 16 and 18 E6 protein with p53. Science. 1990; 248:76–79. PMID: 2157286.
16. Park IC, Park MJ, Woo SH, Lee HC, An S, Gwak HS, et al. Tetraarsenic oxide induces apoptosis in U937 leukemic cells through a reactive oxygen species-dependent pathway. Int J Oncol. 2003; 23:943–948. PMID: 12963972.

17. Park MJ, Park IC, Bae IJ, Seo KM, Lee SH, Hong SI, et al. Tetraarsenic oxide, a novel orally administrable angiogenesis inhibitor. Int J Oncol. 2003; 22:1271–1276. PMID: 12738993.

18. Ahn WS, Bae SM, Lee KH, Kim YW, Lee JM, Namkoong SE, et al. Comparison of effects of As2O3 and As4O6 on cell growth inhibition and gene expression profiles by cDNA microarray analysis in SiHa cells. Oncol Rep. 2004; 12:573–580. PMID: 15289840.
19. Yoo MH, Kim JT, Rhee CH, Park MJ, Bae IJ, Yi NY, et al. Reverse effects of tetraarsenic oxide on the angiogenesis induced by nerve growth factor in the rat cornea. J Vet Med Sci. 2004; 66:1091–1095. PMID: 15472473.

20. Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999; 93:268–277. PMID: 9864170.

21. Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the national cancer institute cooperative research and development studies. Oncologist. 2001; 6(Suppl. 2):22–28. PMID: 11331437.

22. Astudillo H, Lopez T, Castillo S, Gariglio P, Benitez L. p53, Bcl-2, PCNA expression, and apoptotic rates cervical tumorigenesis. Ann N Y Acad Sci. 2003; 1010:771–774. PMID: 15033825.
23. Mitchell KO, Ricci MS, Miyashita T, Dicker DT, Jin Z, Reed JC, et al. Bax is a transcriptional target and mediator of c-myc-induced apoptosis. Cancer Res. 2000; 60:6318–6325. PMID: 11103792.
24. St. John LS, Sauter ER, Herlyn M, Litwin S, Adler-Storthz K. Endogenous p53 gene status predicts the response of human squamous cell carcinoma to wild-type p53. Cancer Gene Ther. 2000; 7:749–756. PMID: 10830722.
25. Takehara T, Takahashi H. Suppression of Bcl-XL deamidation in human hepatocellular carcinomas. Cancer Res. 2003; 63:3054–3057. PMID: 12810626.
Fig. 1
The chemical structures of As2O3 and As4O6. The shaded bigger circles and empty smaller circles represent As and O, respectively.
Fig. 2
Growth inhibition patterns of As2O3 and As4O6 in SiHa cells in vitro. Cells were treated with the indicated amount of the two arsenic compounds, As2O3 and As4O6, over 4 day incubation periods. Cell growth suppression was measured as described in "Methods and Materials." OD was measured at 570 nm. The assay was performed in triplicate, with the average OD values and SD recorded, which was repeated twice more, with similar results. *Statistically significant at p<0.05, using the paired Student's t-test, compared to no drug treatment (control, CTL).
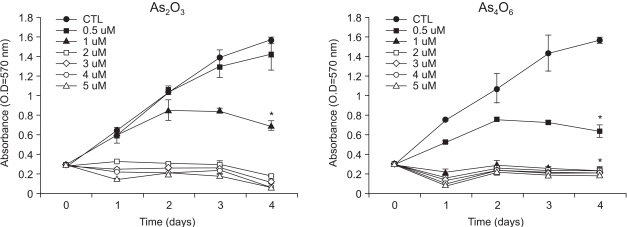
Fig. 3
Induction of DNA ladder formation (A) and sub-G1 cell population (B) in SiHa cells due to treatment with As2O3 and As4O6. Cells were treated with 0.5 and 1 µM of As2O3 or As4O6 for 48 h. (A) DNA was analyzed on a 2% agarose gel and photographed under UV light (Con: Control, SM: Size marker). (B) Cells were stained with propidium iodide, and analyzed using flow cytometer, for the detection of the sub-G1 population.
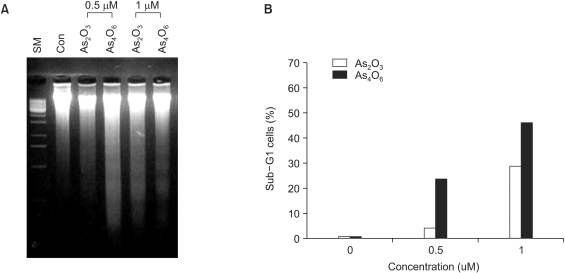
Fig. 4
The induction of early and late apoptotic cells in SiHa cells due to treatment with As2O3 and As4O6. Cells were treated with 0.5 and 1 µM of As2O3 or As4O6 for 48 h. Cells were stained with both annexin V-FITC and propidium iodide, and then analyzed for different apoptotic cell populations using flow cytometry.
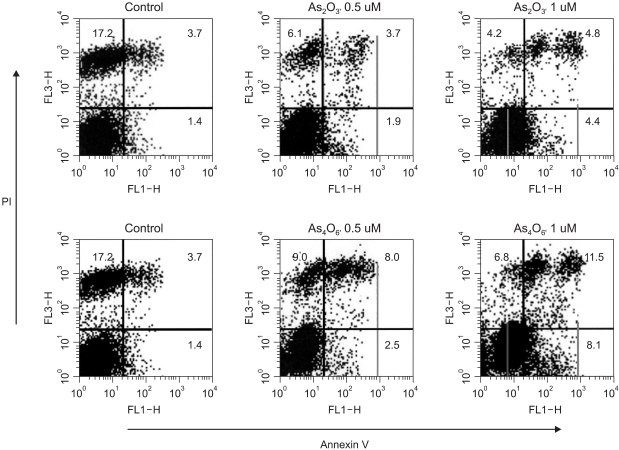
Fig. 5
Western blots of the cell proliferation marker and apoptosis-related proteins in SiHa cells due to treatment with As2O3 and As4O6. Cells were treated with 0.5 and 1 µM of As2O3 or As4O6 for 48 h. The cells were harvested and the cell lysates run on 12% SDS-PAGE. Subsequent protein bands were transblotted onto a nitrocellulose membrane for an immunoblot assay.