Abstract
Purpose
To evaluate the treatment outcomes of the three-dimensional conformal radiotherapy (3D-CRT), in conjunction with induction chemotherapy, for the treatment of stage III non-small cell lung cancer (NSCLC).
Materials and Methods
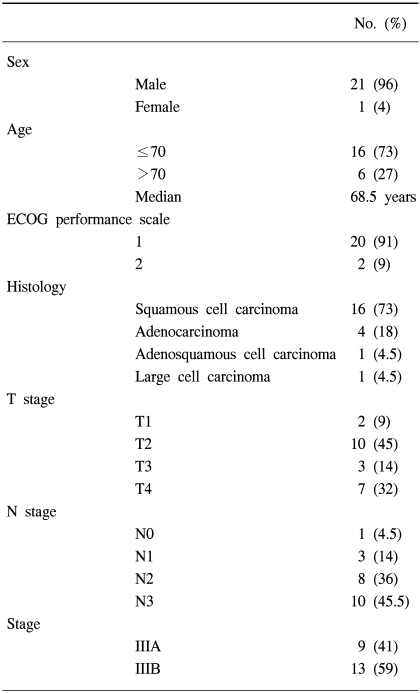
Between November 1998 and March 2003, 22 patients with histologically proven, clinical stage III NSCLC, treated with induction chemotherapy, followed by 3D-CRT, were retrospectively analyzed. There were 21 males (96%) and 1 female (4%), with a median age of 68.5 (range, 42~79). The clinical cancer stages were IIIA and IIIB in 41 and 59%, respectively. The histologies were squamous cell carcinoma, adenocarcinoma and others in 73, 18 and 9%, respectively. Twenty patients (91%) received induction chemotherapy before radiation therapy. The majority of the chemotherapy regimen consisted of cisplatin and gemcitabine. Radiation was delivered with conventional anteroposterior/posteroanterior fields for 36 Gy, and then 3D-CRT was performed. The total radiation dose was 70.2 Gy. The median follow-up period was 17 months (range, 4~59 months).
Results
The median overall survival was 19 months. The two and four-year overall survival rates were 37.9 and 30.3%, respectively. The median progression-free survival was 21 months. The two and four-year progression-free survival rates were 42.1 and 21%, respectively. The prognostic factors for overall survival by a univariate analysis were age, histology and T stage (p<0.05). Acute radiation toxicities, as evaluated by the RTOG toxicity criteria, included two cases of grade 3 lung toxicity and one case of grade 2 esophagus toxicity.
The cure of non-small cell lung cancer (NSCLC) is still far from being reached, largely due to the high rate of distant metastasis (1); therefore, advances in chemotherapy will inevitably be required. However, local control also remains a serious problem, with the local failure rate following definitive radiation therapy still being high (2).
The results of the Radiation Therapy Oncology Group (RTOG) Protocol 73-01 were published in 1980, which suggested that local control was improved by increasing the dose of radiation, and the local failure rate decreased as the dose was increased from 40 to 60 Gy (3). This suggested further improvement in local control with higher doses of radiation. However, conventional radiotherapeutic techniques were limited in their ability to increase the dose of radiation due to the volume of normal lung tissue included in the high dose region.
Three-dimensional conformal radiotherapy (3D-CRT) represents one of the methods designed to improve the delivery of radiation in NSCLC (4,5). While the dose delivered to normal tissues, including the lung, esophagus and heart, is reduced, the target tumor can be more completely and accurately covered in the treatment field. With these advantages, 3D-CRT allows higher radiation dose to be received, with the goal of improving local control.
Radiation therapy, with induction chemotherapy, may help to minimize the overlapping toxicities of the two treatment modalities, while improving the overall outcome (6). There has been limited study in the use of 3D-CRT in conjunction with induction chemotherapy. In this study, our experience of NSCLC treated with this approach is reviewed.
Between November 1998 and March 2003, twenty two patients with histologically confirmed and clinical stage III NSCLC, treated at Chungnam National University Hospital, using induction chemotherapy, followed by 3D-CRT, were included in this study. Those patients with malignant pleural effusion, weight loss >10% during last 6 months, ECOG performance scale >2, and FEV1 ≤1 L were excluded from the 3D-CRT.
The pretreatment characteristics of the twenty two patients are presented in Table 1. The clinical stage was determined using chest CT, bronchoscopy, whole-body bone scan and brain CT or MRI. Stages T3N1 & T1-3N2 were grouped as stage IIIA, and T4 or N3 as stage IIIB. Nine patients (41%) were classed as stage IIIA and thirteen (59%) as stage IIIB. Most of the patients had either a squamous cell carcinoma (73%) or adenocarcinoma (18%), with other histologies including adenosquamous cell carcinomas and large cell carcinomas. The ages of the patients ranged from 42 to 79 years, with a median of 68.5 years. There was a 96% male predominance and ECOG performance scale of 1 in 91%.
Radiation therapy was delivered with a 10 MV photon beam. Initially, 36 Gy/20 fractions, with anteroposterior/posteroanterior fields, were given, followed by 34.2 Gy/19 fractions, with 3D-CRT (total 70.2 Gy).
In the first preparation for radiation therapy, patients were immobilized in the supine position, with their arms raised in a customized alpha-cradle mold or air vacuum cushion. This was followed by a planning CT scan in the treatment position. After the digital image data were transferred into a treatment planning system, the target volumes and normal structures were outlined on each slice by a radiation oncologist.
The pre-chemotherapy tumor volume was considered when choosing target volume, but when a marked reduction of the tumor was shown after induction chemotherapy, the post-chemotherapy tumor volume was also considered. The gross target volume (GTV) included the tumor and lymph nodes that were more than 1 cm, with the clinical target volume (CTV) delineated with a 1 cm margin around the GTV. The planning target volume (PTV) was considered to be the same as the CTV. The margin was further adjusted by checking organ movements with fluoroscopy. The beam's-eye-view (BEV) technique was used to find beam pathways that could wrap around the target volume adequately and; thus, decrease normal tissue damage. Clinically uninvolved lymph nodes were not treated prophylactically, either with the conventional radiation therapy or 3D-CRT.
The number of irradiated fields ranged from five to eight. The radiation dose was prescribed to the isodose level, encompassing the CTV. A dose-volume histogram (DVH) was produced with a review of the CTV, normal lung, heart, spinal cord and esophagus.
The fractionation scheme was 1.8 Gy a day, 5 days a week.
The induction chemotherapy was administered to 20 patients (91%). In 17 patients (85%) of these 20 patients, the combination regimen was cisplatin and gemcitabine (cisplatin 70 mg/m2 on Day 1, gemcitabine 1,250 mg/m2 on Days 1 and 8, repeated every 3 weeks), with the total number of cycles ranging from three to six. The chemotherapy regimens off the protocol were determined according to the preference of the referring medical oncologist.
Three to six weeks after completion of the chemotherapy, the sequential radiation therapy was initiated.
The criteria used to determine the objective treatment response were adapted from the WHO Handbook (7). The treatment response was evaluated mainly with chest CT, but chest PA and bronchoscopy were also used, which were performed two times, one after induction chemotherapy and the other, one to two months after the sequential radiation therapy. Each of the two evaluations of treatment response was based on a comparison to the initial tumor status. Acute radiation toxicities were evaluated by the RTOG toxicity criteria.
After evaluation of the radiation therapy response, patients were seen at follow-up every 3 months for 2 years, then every 4~6 months thereafter.
The overall survival (OS) was calculated from the first day of treatment to the last follow-up, using the Kaplan-Meier method. Progression-free survival (PFS) was from the first day of treatment to the detection of local progression or distant metastasis. A univariate analysis was performed using the logrank test to inspect the prognostic significance of the patients' characteristics and treatment variables.
All twenty two patients finished the scheduled dose of 70.2 Gy, with a median follow-up period of 17 months, ranging from 4 to 59 months.
The median overall survival was 19 months. The two and four-year overall survival rates were 37.9 and 30.3%, respectively. The median progression-free survival was 21 months. The two and four-year progression-free survival rates were 42.1 and 21%, respectively (Fig. 1, 2).
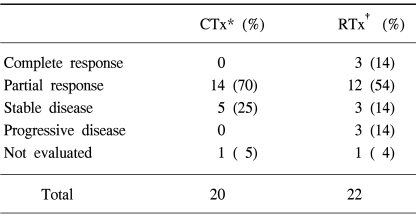
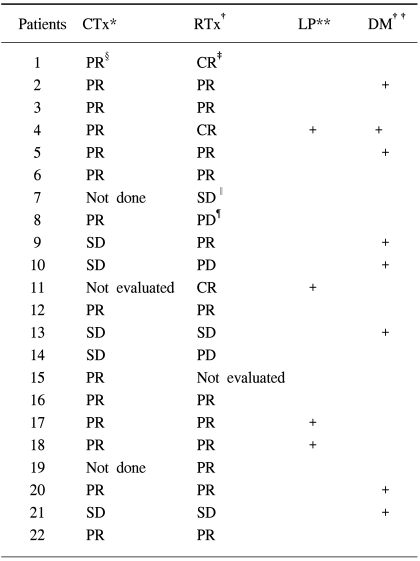
The response evaluation of the induction chemotherapy was not performed in one patient, as he declined to take a chest CT for an unknown reason; another patient could not be evaluated for response to the sequential radiation therapy due to pneumonia of the entire lung. The rates of patients who showed a response to the treatments (complete and partial response) were 70% after the induction chemotherapy and 68% after the sequential radiation therapy (Table 2, 4).
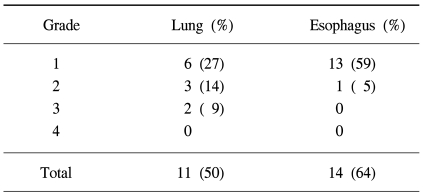
Sixteen (73%) patients suffered acute radiation toxicities. Grade 3 lung toxicity and grade 2 esophagus toxicity occurred in 2 (9%) and 1 patient (5%), respectively (Table 5). All the acute toxicities resolved spontaneously, or with supportive care, after completion of the radiation therapy.
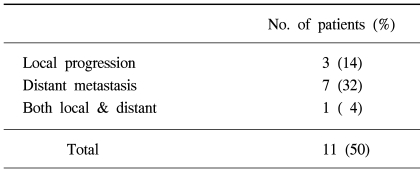
Eleven (50%) patients were found to have disease progression during follow-up. Local progression and distance metastasis occurred in 3 (14%) and 7 patients (32%), respectively, with both occurring in 1 patient (Table 3, 4). The most common site of distant metastasis was the brain, and other sites, in order, included the liver, contralateral lung, adrenal gland and bone. The salvage treatment after detection of disease progression was chemotherapy, and radiation therapy was used for distant metastasis.
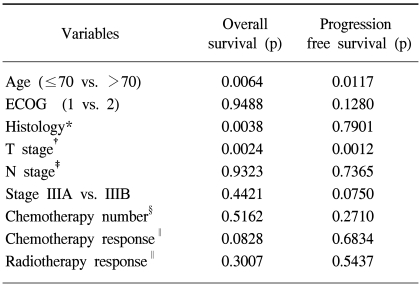
The significant favorable prognostic factors for the overall survival by a univariate analysis were age ≤70 (p=0.0064), histology of squamous cell carcinoma (p=0.0038) and lower T stage (p=0.0024). The univariate analysis also revealed age ≤70 (p=0.0117) and lower T stage (p=0.0012) to be significant prognostic factors for progression-free survival (Table 6).
Over the last three decades, for patients with unresectable and locally advanced NSCLC, the standard radiation therapy was 60 Gy, administered in single daily fractions of 2 Gy (3), but both local failure and distant metastasis were significant problems.
To improve local control, a major obstacle in the cure of NSCLC, there have been many attempts to develop more effective methods of radiation therapy, for example, continuous hyperfractionated accelerated radiation therapy (CHART) or hyperfractionated accelerated radiation therapy (HART); the RTOG initiated dose-escalation trial using hyperfractionation regimen (1.2 Gy, twice a day). A dose of 69.6 Gy with the hyperfractionation regimen showed improved survival compared with 60 Gy daily radiation therapy, but no improvement was found by increasing the dose above 69.6 Gy (8). However, after taking into consideration treatment interruptions, which were more common in higher dose groups, there was an improved 5-year survival among patients without treatment interruptions when treated with >69.6 Gy than <69.6 Gy (15 vs. 7%, respectively, p=0.014) (9). Furthermore, there were no increased toxicities associated with the higher total dose (8).
However, conventional radiotherapy techniques were limited in increasing the radiation dose, due to the amount of normal tissue volume included in the treatment fields. The introduction of 3D-CRT presented a possible solution to this problem. It has subsequently provided more accurate target localization due to the use of complex field arrangements (10), and makes it possible for the radiation oncologist to delineate target contours, choose beam angles and determine dose distributions more accurately than previously. Precise dose-volume calculations can be made for normal structures, which assist in the selection of an ideal treatment plan, which can also permit an escalation of the radiation dose to the primary target site, without increasing toxicities associated with a higher dose when delivered with conventional radiotherapy techniques (11). In recent years, there have been magnificent improvements in computer technology and treatment planning; therefore the use of 3D-CRT has become even more practical (10).
Our results, a median survival of 19 months and 2-year survival rate of 37.9% for stage III NSCLC patients, are comparable to previously documented studies (14~16) (Table 7). The results of our study have demonstrated that the treatment regimen was well tolerated, without excessive grade 3 or 4 acute toxicities.
Several results with definitive 3D-CRT alone have been published for inoperable NSCLC. At a Washington University study, the one and two-year overall survival rates were 59 and 41%, respectively (12). Patients receiving doses ≥70 Gy had better local control and cause-specific survivals than those treated with lower doses (p=0.05), but a poorer overall survival. The results of the study at the University of Michigan with 3D-CRT alone for NSCLC also demonstrated significant improvements in local progression-free survival (LPFS) with doses above 67.6 Gy (13). The reported median survival and 2-year survival rates for stage IIIA patients (14 months and 36%) compared favorably with the RTOG results using 69.6 Gy (8), as well as the combined modality results reported by Dillman et al (14). However, the 25 stage IIIB patients fared significantly worse, with a median survival and 2-year survival rate of 12 months and 17%, respectively. Despite doses of up to 74 Gy, the toxicity was low, with only one episode of grade 3~4 toxicity.
The rationale for using sequential chemotherapy and radiation therapy stems from the observation that the former suppressed distant metastasis (17), and the latter improved local control. The Memorial Sloan-Kettering Cancer Center compared 3D-CRT alone (70.2 Gy) with sequential chemotherapy (platinum based) and 3D-CRT (64.8 Gy) for stage III NSCLC (15). The median survival and 2-year local control rate were significantly increased in the combined-modality group (18.1 vs. 11.7 months, 43.1 vs. 35.4%, respectively), while grade 3 or worse non-hematological toxicities were decreased in the combined modality group (16 vs. 20%, respectively). Considering that more stage IIIB patients were included in the combined- modality group (70 vs. 51%, respectively), chemotherapy appears to be beneficial, even in patients who are receiving a higher dose of radiation therapy than those typically given with conventional techniques. However, the optimal sequence of chemotherapy and radiation therapy in NSCLC remains to be established, and recent studies suggest that a concurrent may be better than a sequential combination (18,19).
Elective nodal irradiation was not included in this study. Rosenzweig et al. published results for 171 patients treated definitely with 3D-CRT to the involved field volumes, without elective nodal irradiation (20). The proportion of patients who had elective nodal failures was only 6.5%, and Senan et al. reported similarly low failure rates for untreated elective nodal areas in stage III patients (21).
Several phase I radiation escalation trials have been performed, and have demonstrated that 74~90 Gy can be safely delivered if strict dose-volume limitations are applied for critical structures (22). Our institution has a plan to escalate the radiation dose up to 80 Gy, with full 3D-CRT, using DVH parameters, such as V20, Veff and total lung mean dose (23). Among the newer approaches evolving from 3D-CRT, intensity-modulated radiation therapy (IMRT) can optimally manipulate the intensities of individual rays within each beam; thus, has the potential to more effectively decrease lung toxicity due to radiation therapy. Also, investigations are under way to either control respiratory motion (24) or to gate the linear accelerator to the 'beam-on-state' during selected phases of the ventilatory cycle (25).
Even with the addition of chemotherapy, local control remains a challenge in the treatment of locally advanced NSCLC, and higher doses of radiation appear to improve local control. Although only a small number of patients, with a relatively short follow-up period are the weak points of this study, it has shown that 3D-CRT can be delivered safely and effectively in conjunction with induction chemotherapy. Further studies need to be performed to determine the optimal radiation dose and sequencing of the chemotherapy and 3D-CRT.
In summary, this study has demonstrated that increasing the radiation dose for NSCLC, using 3D-CRT, does not significantly increase the acute toxicities. Sequential chemoradiotherapy, including 3D-CRT, seems to be a safe, well-tolerated and effective treatment modality for stage III NSCLC.
References
1. Perez CA, Pajak TF, Rubin P, Simpson JR, Mohiuddin M, Brady LW, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy: a report by the Radiation Therapy Oncology Group. Cancer. 1987; 59:1874–1881. PMID: 3032394.
2. Le Chevalier T, Arriagada R, Quoix E, Ruffie P, Martin M, Tarayre M, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991; 83:417–423. PMID: 1847977.

3. Perez C, Stanley K, Rubin P, Kramer S, Brady L, Perez-Tamayo R, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung: preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980; 45:2744–2753. PMID: 6991092.

4. Armstrong J, Raben A, Zelefsky M, Burt M, Leibel S, Burman C, et al. Promising survival with three-dimensional conformal radiation therapy for non small cell lung cancer. Radiother Oncol. 1997; 44:17–22. PMID: 9288852.
5. Robertson JM, Ten Haken RK, Hazuka MB, Turrisi AT, Martel MK, Pu AT, et al. Dose escalation for non-small cell lung cancer using conformal radiation therapy. Int J Radiat Oncol Biol Phys. 1997; 37:1079–1085. PMID: 9169816.

6. Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995; 311:899–909. PMID: 7580546.
7. World Health Organization Offset Publication No.48. WHO handbook for reporting results of cancer treatments. 1979. Geneva (Switzerland):
8. Cox JD, Azarnia N, Byhardt RW, Shin KH, Emami B, Pajak TF. A randomized phase I/II trial of hyperfractionated radiation therapy with total doses of 60.0 Gy to 79.2 Gy: possible survival benefit with 69.6 Gy in favorable patients with Radiation Therapy Oncology Group stage III non small cell lung carcinoma: report of Radiation Therapy Oncology Group 83-11. J Clin Oncol. 1990; 8:1543–1555. PMID: 2167952.
9. Cox JD, Pajak TF, Asbell S, Russell AH, Pederson J, Byhardt RW, et al. Interruptions of high-dose radiation therapy decrease long-term survival of favorable patients with unresectable non-small cell carcinoma of the lung: Analysis of 1244 cases from 3 Radiation Therapy Oncology Group (RTOG) trials. Int J Radiat Oncol Biol Phys. 1993; 27:493–498. PMID: 8226140.

10. Vijayakumar S, Low N, Chen GT, Myrianthopoulos L, Culbert H, Chiru P, et al. Beam's Eye View-based photon radiotherapy I. Int Radiat Oncol Biol Phys. 1991; 21:1575–1586.

11. Myrianthopoulos LC, Chen GT, Vijayakumar S, Halpern HJ, Spelbring DR, Pelizzari CA. Beam's eye View volumetrics: an aid in rapid treatment plan development and evaluation. Int J Radiat Oncol Biol Phys. 1992; 23:367–375. PMID: 1587758.

12. Bradley JD, Ieumwananothachai N, Purdy JA, Wasserman TH, Lockett MA, Graham MV, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2002; 52:49–57. PMID: 11777621.
13. Hazuka MB, Turrisi AT 3rd, Lutz ST, Martel MK, Ten Haken RK, Strawderman M, et al. Results of high-dose thoracic irradiation incorporating beam's eye view display in non-small cell lung cancer: a retrospective multivariate analysis. Int J Radiat Oncol Biol Phys. 1993; 27:273–284. PMID: 8407401.

14. Dillman RO, Seagren SL, Propert KJ, Guerra J, Eaton WL, Perry MC, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990; 323:940–945. PMID: 2169587.

15. Dillman RO, Herndon J, Seagren SL, Eaton WL Jr, Green MR. Improved survival in stage III non-small cell lung cancer: seven-year follow-up of Cancer and Leukemia Group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996; 88:1210–1215. PMID: 8780630.
16. Sim S, Rosenzweig KE, Schindelheim R, Ng KK, Leibel SA. Induction chemotherapy plus three-dimensional conformal radiation therapy in the definitive treatment of locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2001; 51:660–665. PMID: 11597806.

17. Paek CW, Yun SY, Seo JH, Choi CW, Kim BS, Shin SW, et al. Concurrent chemoradiation therapy with cisplatin and oral etoposide for locally advanced non-small cell lung cancer. J Korean Cancer Assoc. 2000; 32:682–689.
18. Curran WJ, Scott C, Langer C, Komaki R, Lee JS, Hauser S, et al. Phase III comparison of sequential vs. concurrent chemoradiation for patients with unresected stage III non-small cell lung cancer (NSCLC): initial report of Radiation Therapy Oncology Group (RTOG) 9410. Proc Am Soc Clin Oncol. 2000; 19:1891a.
19. Rosenzweig KE, Sim SE, Mychalczak B, Braban LE, Schindelheim R, Leibel SA. Elective nodal irradiation in the treatment of non-small cell lung cancer with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2001; 50:681–685. PMID: 11395236.
20. Senan S, Burgers S, Samson MJ, van Klaveren RJ, Oei SS, van Sornsen de Koste J, et al. Can elective nodal irradiation be omitted in stage III non-small-cell lung cancer? Analysis of recurrences in a phase II study of induction chemotherapy and involved-field radiotherapy. Int J Radiat Oncol Biol Phys. 2002; 54:999–1006. PMID: 12419425.

21. Sibley GS, Mundt AJ, Shapiro C, Jacobs R, Chen G, Weichselbaum R, et al. The treatment of stage III non small cell lung cancer using high dose conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1995; 33:1001–1007. PMID: 7493826.
22. Socinski MA, Morris DE, Halle JS, Moore DT, Hensing TA, Limentani SA, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol. 2004; 22:4341–4350. PMID: 15514375.

23. Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1999; 45:323–329. PMID: 10487552.

24. Cheung PC, Sixel KE, Tirona R, Ung YC. Reproducibility of lung tumor position and reduction of lung mass within the planning target volume using active breathing control (ABC). Int J Radiat Oncol Biol Phys. 2003; 57:1437–1442. PMID: 14630283.

25. Berson AM, Emery R, Rodriguez L, Richards GM, Ng T, Sanghavi S, et al. Clinical experience using respiratory gated radiation therapy: comparison of free-breathing and breathhold techniques. Int J Radiat Oncol Biol Phys. 2004; 60:419–426. PMID: 15380575.

Fig. 1
Overall survival. The median overall survival was 19 months. The two and four-year overall survival rates were 37.9 and 30.3%, respectively.
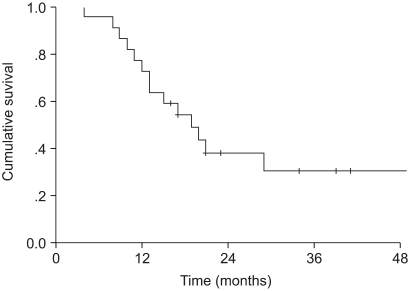
Fig. 2
Progression-free survival. The median progression-free survival was 21 months. The two and four-year progression-free survival rates were 42.1 and 21%, respectively.
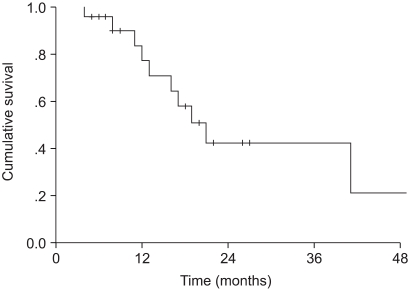
Table 7
Comparison of results
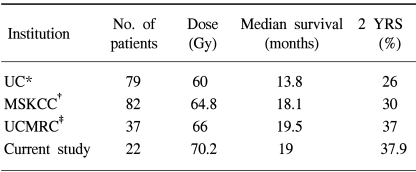
*university of California (Dillman et al), stage I~III patients, induction chemotherapy+conventional radiotherapy, †memorial sloan-kettering cancer center, stage III patients, induction chemotherapy+ 3D-CRT, ‡university of Chicago Michael Reese Center, stage III patients, induction or concurrent or adjuvant chemotherapy+ 3D-CRT.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download