1. Russo IH, Russo J. Hormonal approach to breast cancer prevention. J Cell Biochem. 2000; 77(S34):1–6. PMID:
10679811.

2. Gupta V, Harkin DP, Kawakubo H, Maheswaran S. Transforming growth factor-β superfamily: evaluation as breast cancer biomarkers and preventive agents. Curr Cancer Drug Targets. 2004; 4:165–182. PMID:
15032667.
3. Di Loreto C, Reis FM, Cataldi P, Zuiani C, Luisi S, Beltrami CA, et al. Human mammary gland and breast carcinoma contain immunoreactive inhibin/activin subunits: evidence for a secretion into cystic fluid. Eur J Endocrinol. 1999; 141:190–194. PMID:
10427163.

4. Zheng W, Lauchlan SC. Inhibin and activin: their roles in ovarian tumorigenesis and their diagnostic utility in surgical pathology practice. Appl Immuno & Mol Morph. 1999; 7:29–38.

5. Robertson DM, Pruysers E, Burger HG, Jobling T, McNeilage J, Healy D. Inhibins and ovarian cancer. Mol Cell Endocrinol. 2004; 225:65–71. PMID:
15451569.

6. Reis FM, Luisi S, Carneiro MM, Cobellis L, Federico M, Camargos AF, et al. Activin, inhibin and the human breast. Mol Cell Endocrinol. 2004; 225:77–82. PMID:
15451571.

7. Reimer T, Koczan D, Muller H, Friese K, Krause A, Thiesen HJ, et al. Human chorionic gonadotrophin-β transcripts correlate with progesterone receptor values in breast carcinomas. J Mol Endocrinol. 2000; 24:33–41. PMID:
10656995.
8. Janssens JP, Verlinden I, Gungor N, Raus J, Michiels L. Protein biomarkers for breast cancer prevention. Eur J Cancer Prev. 2004; 13:307–317. PMID:
15554559.
9. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410. PMID:
1757079.

10. O'Malley FP, Bane AL. The spectrum of apocrine lesions of the breast. Adv Anat Pathol. 2004; 11:1–9. PMID:
14676636.
11. Risbridger GP, Ball EM, Wang H, Mellor SL, Peehl DM. Re-evaluation of inhibin alpha subunit as a tumour suppressor in prostate cancer. Mol Cell Endocrinol. 2004; 225:73–76. PMID:
15451570.
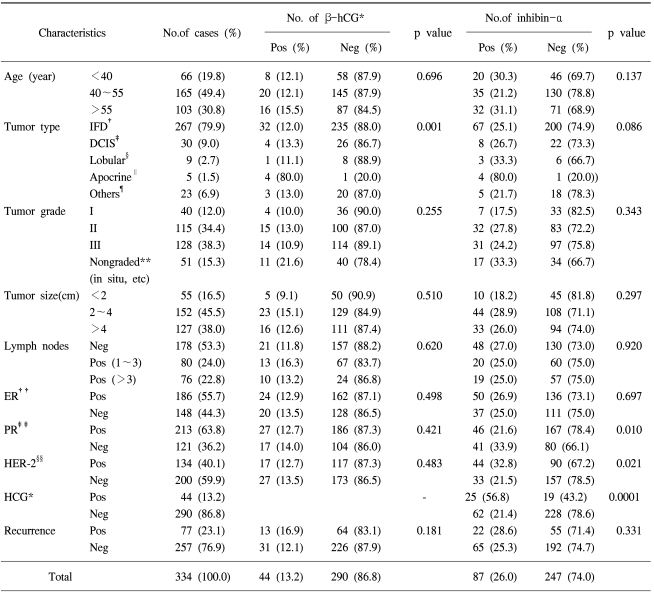
12. Agnantis NJ, Patra F, Khaldi L, Filis S. Immunohistochemical expression of subunit beta HCG in breast cancer. Eur J Gynaecol Oncol. 1992; 13:461–466. PMID:
1282101.
13. Wachner R, Wittekind C, von Kleist S. Immunohistological localization of beta-HCG in breast carcinomas. Eur J Cancer Clin Oncol. 1984; 20:679–684. PMID:
6203754.
14. Chung TS, Kim BG, Cha SJ, Park SI, Lim HM, Park SJ, et al. Expression of topoisomerase II-α and c-erbB-2 in breast cancer. J Korean Breast Cancer Soc. 2002; 5:135–141.

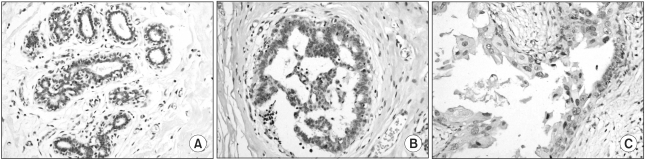
15. McCluggage WG, Maxwell P. Adenocarcinomas of various sites may exhibit immunoreactivity with anti-inhibin antibodies. Histopathology. 1999; 35:216–220. PMID:
10469213.

16. Robertson DM, Stephenson T, Cahir N, Tsigos A, Pruysers E, Stanton PG, et al. Development of an inhibin alpha subunit ELISA with broad specificity. Mol Cell Endocrinol. 2001; 180:79–86. PMID:
11451575.
17. Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, et al. Betaglycan binds inhibin and can mediate functional antagonism of activin signaling. Nature. 2000; 404:411–414. PMID:
10746731.
18. Kuida CA, Braunstein GD, Shintaku P, Said JW. Human chorionic gonadotropin expression in lung, breast, and renal carcinomas. Arch Pathol Lab Med. 1988; 112:282–285. PMID:
2449877.
19. Guo S, Russo IH, Lareef MH, Russo J. Effect of human chorionic gonadotrophin in the gene expression profile of MCF-7 cells. Int J Oncol. 2004; 24:399–407. PMID:
14719117.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download