Abstract
Purpose
Fluorouracil (5-FU) and leucovorin combination therapy have shown synergistic or additive effect against advanced colorectal cancer, but the frequency of mucositis and diarrhea is increased. Most previous studies have used high dose leucovorin (300~500 mg/m2). However, some studies of oxaliplatin and 5-FU with low-dose or high-dose leucovorin in Korea have shown similar response rates. Therefore, we studied the necessity of leucovorin and evaluated the objective tumor response rates and toxicities of a regimen of oxaliplatin and 5-FU without leucovorin every 2 weeks in metastatic colorectal cancer patients.
Materials and Methods
Twenty-four patients with metastatic colorectal cancer were enrolled between January 2002 and March 2003. Patients received 85 mg/m2 of oxaliplatin on day 1, a bolus 5-FU 400 mg/m2 on day 1 and a continuous 5-FU infusion at 600 mg/m2/ 22 hours days 1 and 2, every 2 weeks.
Results
Of the 24 patients treated, 17 patients received previous 5FU with leucovorin and/or other chemotherapy. Three patients could not be evaluated. Five partial responses were observed with overall response rate of 21% (n=24). Of the previous chemotherapy group (n=17), 4 partial responses were observed with response rate of 24%. Median overall survival was 18 months (range 4~32 months) and median progression free survival was 4 months (range 2~6 months). This regimen was well tolerated and only 1 grade 3 anemia was observed.
Go to : 
In 2002, approximately 11,000 people were newly diagnosed with colorectal cancer, which ranked as the 8.1% leading cause of death in Korea (1). For years, the treatment of metastatic colorectal cancer was limited to flurouracil (5-FU), but the effectiveness of single therapy was represented by poor response rates and dismal survival. The introduction of biochemical modulators such as leucovorin or levamisole improved the response rate, but overall survival and progression free survival have remained unaffected. And various modifications of the 5-FU infusion schedule, such as continuous infusion or chronomodulation, have resulted in only borderline improvement (2).
Especially in the comparison study of 5-FU alone versus 5-FU with high-dose or low-dose leucovorin, only the high- dose leucovorin regimen displayed a significant improvement in response rates which was not seen with low dose leucovorin (3). Additionally, the 5-FU and leucovorin regimen was associated more frequently with mucositis and diarrhea than the 5-FU alone treatment regimen (3,4).
Oxaliplatin forms reactive platinum complexes, which are believed to inhibit DNA replication and transcription by forming interstrand and intrastrand cross-linking of DNA molecules. In vitro oxaliplatin inhibits colorectal tumor cell lines resistant to cisplatin and carboplatin (5). Response rates as a single agent in metastatic colorectal cancer reached 10~24% in phase II trials (6,7).
In previous FOLFOX studies, oxaliplatin in combination with 5-FU and leucovorin usually used the high dosage of leucovorin (200~500 mg/m2), and had shown good therapeutic response in metastatic colorectal cancer patients who were previously treated. For many years, Korean medical insurance only allowed low dose leucovorin (20 mg/m2) therapy. The cost of high dose leucovorin and low dose leucovorin in the oxaliplatin plus 5-FU with leucovorin regimen was approximately 200,000 and 20,000 won lespectively. However, in the previous FOLFOX studies in Korea, the effects of oxaliplatin/5-FU with high-dose and with low-dose leucovorin were similar (8). Therefore, we studied the necessity of leucovorin and evaluated the objective tumor response rates and toxicities to a regimen of oxaliplatin and 5-FU without leucovorin every 2 weeks on metastatic colorectal cancer patients.
Go to : 
Patients were required to have histologically proven colorectal adenocarcinoma, bidimensionally measurable lesions, ECOG performance status less than or equal to 2, adequate bone marrow, renal and hepatic function. Clinical, biologic, and radiologic assessments had to be performed before the start of treatment and after the treatment had commenced. We retrospectively reviewed the charts of these patients.
Chemotherapy consisted of oxaliplatin (85 mg/m2 on day 1) as a 2-h infusion followed by bolus 5-FU (400 mg/m2 on day 1), and 5-FU 24-hour infusion 600 mg/m2 on day 1 and 2. Treatments were repeated every two weeks. Treatment was delayed for up to 2 weeks if the absolute number of neutrophils was lower than 1,500/µl; platelets count was lower than 100,000/µl. The 5-FU dose was reduced by 25% for subsequent courses after National Cancer Institute Common Toxicity Criteria (NCI-CTC) grade 3 diarrhea, or mucositis had occurred. The dose of oxaliplatin was reduced by 25% in subsequent cycles if there was NCI-CTC grade 2 neurotoxicity. If NCI-CTC grade 3 neurotoxicity was occurred, treatment was discontinued. Treatments were continued until there were disease progression, unacceptable toxic effects, or the patient refused further treatment.
Before the first and each treatment courses, a physical examination including a neurologic examination, routine hematology and biochemistry analyses, and chest x-ray were performed. The serum CEA levels were determined every two cycles if the CEA was previously elevated. CT scans were performed to define the extent of the disease in the pretreatment evaluation. Tumor size was measured by CT scan, x-ray, or any other technique every 4 cycles or sooner if there was evidence of any clinical deterioration. Patients were assessed before starting each 2 weeks cycle using the NCI-CTC.
Anti-tumor activity was evaluated according to the WHO criteria (9), and dose intensity was calculated as the total cumulative dose divided by duration of dosing. The relative dose intensity was calculated as the dose-intensity divided by the planned dose-intensity, multiplied by 100.
Survival times were calculated from the start of the study treatment until death. Progression free survival was calculated from the first day of the chemotherapy to the date of progressive disease. Progression free survival and overall survival curves were obtained using the Kaplan-Meier method. Response duration was calculated from the date of response confirmation to the date of disease progression.
Go to : 
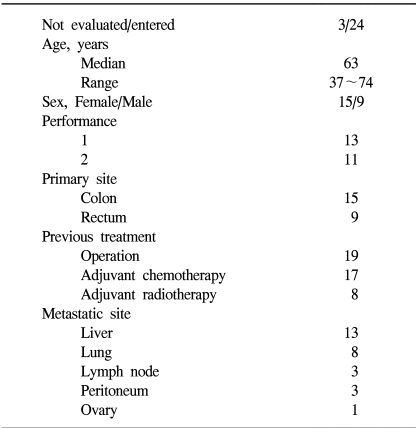
Between January 2002 and March 2003, 24 metastatic colorectal cancer patients received the study drugs at St. Vincent's Hospital. Three patients could not be evaluated for this study, because two patients were lost to follow up and 1 patient died, which was not drug related. The patient's characteristics are listed in Table 1. In the patients with previous adjuvant therapy, 14 patients have relapsed within 12 months and 3 patients have relapsed after 12 months of the last dose of adjuvant therapy.
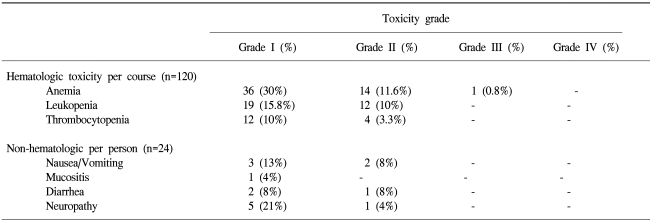
A total of 120 cycles were evaluated for toxicity, and the incidence of toxicity was summarized in Table 2. This regimen was well tolerated and only one grade 3 anemia was observed. The most common non-hematologic toxicities were neurotoxicity, nausea/ vomiting, and diarrhea. One patient died due to pneumonia after a single cycle, however she did not have neutropenia. There were no treatment-related deaths in this study. The relative dose intensities of oxaliplatin and 5-FU were 99.2% and 99.2% of the planned doses.
The median number of cycles was 6 (range 1~9). Of the 21 evaluated patients, 5 patients achieved a partial response, 7 patients had stable disease, and 9 patients had progressive disease. The response rate was 21% by the intention to treat analysis, and 24% by the per protocol analysis. The median overall survival was 18 months (range 4~32 months), and the median progression free survival was 4 months (range 2~6 months). The response rate of untreated group (n=7) was 14%, and the response rate of previous treat group (n=17) was 24%. The median progression free survival of untreated group and previous treated group was same (4 months). Relapsed patients within 12 months of the last dose of adjuvant therapy (n=14) had a 14% response rate.
Go to : 
5FU and leucovorin modulation increases the response rate but has no major impact on survival (2). Presently, the monthly 5-day bolus Mayo Clinic regimen is commonly used as a control treatment in phase III trials (10). In a previous trial, a bimonthly schedule of leucovorin and bolus-plus-infusion 5FU proved superior in response rate (32.6% v 14.5%), progression free survival (27.6 v 22.0 weeks), and toxicity (grade 3 or 4 in 11.1% v 22.9%) than the Mayo Clinic regimen (11).
The addition of oxaliplatin to 5-FU and leucovorin doubled the response rate and progression-free survival among with metastatic colorectal cancer (12). The response rates of oxaliplatin, 5-FU, and leucovorin regimens in chemotherapy naive metastatic colorectal carcinoma were 30~51% (8,12,13). Among these regimens, a bimonthly oxaliplatin, high leucovorin (200 mg/m2) and bolus-plus-infusion 5-FU regimens (FOLFOX 4) in first line therapy had high response rate of 50.7% and long progression free survival of 9 months (12). However, medical insurance in Korea allowed using low dose leucovorin (20 mg/m2) in oxaliplatin and 5FU with leucovorin regimens. Biweekly oxaliplatin combined with low dose leucovorin (20 mg/m2) and bolus and continuous infusion 5-FU as first line therapy had response rate of 40% and progression free survival of 6.6 months in Korea (8).
In the 5-FU and leucovorin resistant metastatic colorectal carcinoma, the response rates of oxaliplatin, 5-FU, and leucovorin regimens were 18~46% (14~18). Among these regimens, a bimonthly oxaliplatin, high-dose leucovorin (200 mg/m2) and 5-FU infusion regimens (FOLFOX3, FOLFOX 4) in 5-FU pretreated colorectal carcinoma had a response rate of 20.6% and progression free survival of 4.7 months (18).
In Korea, four studies of oxaliplatin combined with leucovorin and 5-FU in 5-FU pretreated metastatic colorectal carcinoma were performed. The studies of oxaliplatin (150 mg/m2) combined with the Mayo Clinic regimen {leucovorin (20 mg/m2) and 5-FU (425 mg/m2) for 5 days} repeated in 4-week and oxaliplatin (130 mg/m2) combined with 5-FU (500 mg/m2) bolus followed continuous infusion 5-FU (3000 mg/m2) and leucovorin (100 mg/m2) had each response rate of 25%, 27% and progression free survival of 6.3, 6 months (19,20).
Biweekly oxaliplatin (85 mg/m2) combined with leucovorin (150 mg/m2) and 5-FU (400 mg/m2 bolus and 2,400~1,000 mg/m2 infusion, D1-2) regimen as second line therapy had response rate of 28% and progression free survival of 5.9 months (21). Additionally, biweekly oxaliplatin (85 mg/m2) combined with leucovorin (20 mg/m2) and 5-FU (1,200 mg/m2 infusion, D1-2) had response rate of 42% and progression free survival of 4 months (22).
Our study showed response rates of a previous treat group of 24% and median progression free survival of 4 months. The results of this study demonstrate that oxaliplatin and 5-FU without leucovorin bimonthly regimen showed relatively similar response rate and progression free survival in previous studies which used high-dose and low-dose leucovorin (14~22).
In the view of toxicity, the 5-FU and leucovorin regimen was characterized by more frequent mucositis and diarrhea than the 5-FU only regimen (3,4). Our study showed a very tolerable toxicity profile. The toxicity and tolerability profiles of the regimens evaluated in this study were similar in previous studies, but with lower intensity to those reported by others studies (19~22).
Go to : 
References
1. 2002 Annual report of Korea Central Cancer Registry (published in 2003). Ministry Health and Welfare. Available from URL:
http://www.ncc.re.kr.
2. Schmoll HJ, Buchele T, Grothey A, Dempke W. Where do we stand with 5-fluorouracil? Semin Oncol. 1999; 26:589–605. PMID: 10606252.
3. Petrelli N, Douglass HO Jr, Herrera L, Russell D, Stablein DM, Bruckner HW, et al. Gastrointestinal Tumor Study Group. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. J Clin Oncol. 1989; 7:1419–1426. PMID: 2674331.
4. Doroshow JH, Multhauf P, Leong L, Margolin K, Litchfield T, Akman S, et al. Prospective randomized comparison of fluorouracil versus fluorouracil and high-dose continuous infusion leucovorin calcium for the treatment of advanced measurable colorectal cancer in patients previously unexposed to chemotherapy. J Clin Oncol. 1990; 8:491–501. PMID: 2407810.

5. Rixe O, Ortuzar W, Alvarez M, Parker R, Reed E, Paull K, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem Pharmacol. 1996; 52:1855–1865. PMID: 8951344.
6. Machover D, Diaz-Rubio E, de Gramont A, Schilf A, Gastiaburu JJ, Brienza S, et al. Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann Oncol. 1996; 7:95–98. PMID: 9081400.

7. Becouarn Y, Ychou M, Ducreux M, Borel C, Bertheault-Cvitkovic F, Seitz JF, et al. A phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. J Clin Oncol. 1998; 8:2739–2744. PMID: 9704726.
8. Kwon HC, Kim KT, Lee SA, Park JS, Kim SH, Kim JS, et al. Oxaliplatin with biweekly, low dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFOX 4) as first-line therapy for patients with metastatic colorectal cancer. Cancer Res Treat. 2004; 36:115–120.

9. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47:207–214. PMID: 7459811.

10. Poon MA, O'Connell MJ, Moertel CG, Wieand HS, Cullinan SA, Everson LK, et al. Biochemical modulation of flurouracil: Evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989; 7:1407–1418. PMID: 2476530.
11. de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, et al. Randomized trial comparing monthly low-dose leucovorin and flurouracil bolus with bimonthly high dose leucovorin and flurouracil bolus plus continuous infusion for advanced colorectal cancer: A Frech intergroup study. J Clin Oncol. 1997; 15:808–815. PMID: 9053508.
12. de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000; 18:2938–2947. PMID: 10944126.

13. Levi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997; 350:681–686. PMID: 9291901.
14. de Gramont A, Vignoud J, Tournigand C, Louvet C, Andre T, Varette C, et al. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer. 1997; 33:214–219. PMID: 9135491.

15. de Gramont A, Tournigand C, Louvet C, Andre T, Molitor JL, Raymond E, et al. Oxaliplatin, folinic acid and 5-fluorouracil (folfox) in pretreated patients with metastatic advanced cancer. Rev Med Interne. 1997; 18:769–775. PMID: 9500010.
16. Maindrault-Goebel F, Louvet C, Andre T, Carola E, Lotz JP, Molitor JL, et al. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). Eur J Cancer. 1999; 35:1338–1342. PMID: 10658524.

17. Andre T, Louvet C, Raymond E, Tournigand C, de Gramont A. Bimonthly high-dose leucovorin, 5-fluorouracil infusion and oxaliplatin (FOLFOX3) for metastatic colorectal cancer resistant to the same leucovorin and 5-fluorouracil regimen. Ann Oncol. 1998; 9:1251–1253. PMID: 9862058.
18. Andre T, Bensmaine MA, Louvet C, Francois E, Lucas V, Desseigne F, et al. Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and flurouracil regimen. J Clin Oncol. 1999; 17:3560–3568. PMID: 10550155.
19. Bae YZ, Jung JH, Moon CH, Kim SH, Kwon HC, Kim JS, et al. A phase II study of oxaliplatin combined with 5-Fluorouracil and leucovorin (Mayo clinic regimen) in 5-Fluororuacil Refractory Colorectal Cancer. Cancer Res Treat. 2002; 34:218–222.
20. Bang SM, Cho EK, Oh JH, Chang HM, Ahn JS, Lee JA, et al. Combination chemotherapy of oxaliplatin, 5-flurouracil, and leucovorin in 5-fluorouracil-pretreated patients with metastatic colorectal cancer. Cancer Res Treat. 2003; 33:414–419.
21. Lee KS, Lee WS, Kim HK, Jeong JY, Heo DS, Bang YJ, et al. A phase II study of oxaliplatin, 5-fluorouracil, and leucovorin in 5-Fluorouracil pretereated metastatic colorectal cancer. J Korean Cancer Assoc. 2001; 33:99–105.
22. Lee JH, Lee JH, Kim TW, Lee KH, Kang YK, Lee JS, et al. Combination of oxaliplatin, fluorouracil, and leucovorin in the treatment of fluoropyrimidine-pretreated patients with metastatic colorectal cancer. J Korean Med Sci. 2001; 16:69–74. PMID: 11289404.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download