Abstract
Purpose
To determine the effects of combinations of radiation and flavopiridol, an inhibitor of cyclin-dependent kinases and global transcription, in a human uterine cervix cancer cell line.
Materials and Methods
Human uterine cervix cancer cells (HeLa), cultured to the mid-log phase, were exposed to X-rays, flavopiridol, and combinations of X-rays and flavopiridol in various sequences. The end point in this study was the clonogenic survival, which was measured via clonogenic assays. In order to determine the intrinsic cytotoxicity of flavopiridol, 0, 5, 12.5, 25, 37.5, 50 and 100 nM of flavopiridol were added to cell culture media. In the combination treatment, four different schedules of flavopiridol and irradiation combinations were tested: treatment of flavopiridol for 24 hours followed by irradiation, simultaneous administration of flavopiridol and irradiation, and irradiation followed by flavopiridol (for 24 hours) at intervals of 6 and 24 hours. The fraction of cells surviving after the combination treatment with 2 Gy of radiation (SF2) was compared with that of the fraction of cells surviving after treatment with irradiation alone.
Results
The cytotoxicity of flavopiridol was found to be dose-dependent, with an IC50 of 80 nM. No cytotoxic enhancements were observed when flavopiridol and radiation were administered simultaneously. Flavopiridol, administered either 24 hours before or 6 hours after irradiation, exerted no sensitizing effects on the cells. Only one protocol resulted in a radiosensitizing effect: the administration of flavopiridol 24 hours after irradiation.
Cell cycle progression in mammalian cells is controlled by both cyclins and cyclin-dependent kinases. In cancer cells, these cell cycle-regulatory proteins are frequently expressed abnormally (1,2). Targeting these molecules may, in some cases, improve cancer cells' responses to chemotherapy or radiotherapy
Flavopiridol is a synthetic flavonoid derived from a plant, Dysoxylum binectariferum, which is indigenous to India. Its anticancer effects were identified as the result of drug screening by the National Cancer Institute (NCI) on 60 human cancer cell lines (3). Flavopiridol is a potent inhibitor of cyclin-dependent kinases (cdks); via direct binding to the ATP binding site, flavopiridol inhibits cdk-1 (cdc-2), -2, -4, -6 and -7 at nanomolar concentrations (4~7), and the direct inhibition of cdk activity, flavopiridol arrests cell growth at either the G1/S or G2/M phases of the cell cycle (8). Flavopiridol also results in the indirect inhibition of cell proliferation by reducing the expression levels of cyclin D1, p21Waf1/Cip1 and p27Kip1 (9~13). Flavopiridol has also been demonstrated to induce apoptosis, inhibit angiogenesis and potentiate the effect of chemotherapeutic agents in a variety of in vitro and in vivo models (14~17).
Although there is abundant data regarding the combination of flavopiridol with chemotherapy, only a small amount of data currently exists regarding the combination of flavopiridol with radiation. Raju et al. investigated the in vitro radiosensitizing effect of flavopiridol in the murine ovarian cancer cell line, OCA-I (18) and reported a radiosensitizing effect. The mechanism underlying this phenomenon was thought to involve cell cycle redistribution, and the blocking of sublethal DNA damage repair processes. Jung et al. reported an increased radiosensitivity of gastric and colon cancer cell lines as a result of flavopiridol treatment, and also determined it to be sequence and time-dependent (19). A possible mechanism for flavopiridol's radiosensitizing effects involves the reduction of p21 expression, and the augmentation of apoptotic rates.
In this study, we attempted to characterize the radiosensitizing effects of flavopiridol on a human cervix cancer cell line.
The human uterine cervix cancer cell line (HeLa) used in this study was obtained from the Korean Cell Line Bank. These cells were cultured at 37℃, and 5% CO2 in DMEM (Join Bio Innovation, South Korea) supplemented with 10% fetal bovine serum (JRH Biosciences Inc., Lenexa, KS) and 12.5µg/ml gentamycin (Gibco, Grand Island, NY). Cells were routinely passaged twice a week, using 0.05% trypsin-EDTA.
Mid-log phase cells from monolayer cultures were trypsinized and plated at a density of 200 cells per 60 mm dish. The cells were then incubated for 24 hours prior to treatment. The flavopiridol was kindly provided by Aventis Pharma, and was prepared as a 1 mM stock solution in DMSO prior to use. 0, 5, 12.5, 25, 37.5, 50 and 100 nM concentrations of flavopiridol were added to the medium. After an additional 24 hours, the medium was removed, and the cells then underwent an additional 10 days of incubation at 37℃ with fresh drug-free media, resulting in the formation of colonies. The cells were then fixed with methanol, and stained with 0.5% crystal violet in methanol. Colonies with more than 50 cells were counted, and the surviving fractions (SF) calculated.
In the combination treatments, the flavopiridol was added to the media at a concentration of 75 nM, with the media replaced after 24 hours. Irradiation was administered with 4 MV X-rays generated by a linear accelerator (Clinac 4/100, Varian, Palo Alto, CA), with doses of 0, 2, 5 and 10 Gy, at a dose rate of 2.46 Gy/min.
Known numbers of cells were plated in 25 cm2 flasks, and then incubated for 24 hours. Four different schedules of the combination treatment were then conducted. Cells were irradiated and treated with flavopiridol, either simultaneously or sequentially. In the sequential treatments, flavopiridol was administered for 24 hours, either 24 hours before or after irradiation. Another sequential treatment schedule involved irradiation, followed 6 hours later by 24 hours of flavopiridol treatment only.
The cells were then incubated for 10 days, and staining conducted, as described in section 2 'Cytotoxicity of flavopiridol'(Materials and Methods).
All experiments were carried out in triplicate and repeated twice, unless otherwise indicated. The SF means and standard errors were calculated. Comparisons of the SF2 values of the flavopiridol-treated and control groups were conducted using the SAS t-test (SAS Institute, Inc.). Radiation survival data were fitted to a linear-quadratic model, via a nonlinear regression using the JMP 5.1.2 software (SAS Institute, Inc., Cary, NC). The Sensitizer enhancement ratio (SER) was defined as the ratio of radiation dose required to obtain given SF values in the absence of flavopiridol, to that required to obtain the same SF value in the presence of flavopiridol. The radiation dosage required was calculated from the linear-quadratic model, fitted to the experimental data, as described above.
The SF values of the HeLa cells, according to flavopiridol concentration, are shown in Fig. 1. The cytotoxicity of flavopiridol was found to be dose-dependent. The concentration of flavopiridol required to kill 50% of cells (IC50) was 80 nM.
The SF values of cells treated simultaneously with flavopiridol (75nM) and radiation were compared with those of cells treated with radiation only. The cell survival curves are shown in Fig. 2. The SF2 values of the flavopiridol-treated and untreated cells were 0.531±0.120 and 0.670±0.024, respectively (mean±standard error), which was determined to not be a statistically significant difference (p=0.30). Flavopiridol did not enhance the radiation effects in the context of this treatment protocol.
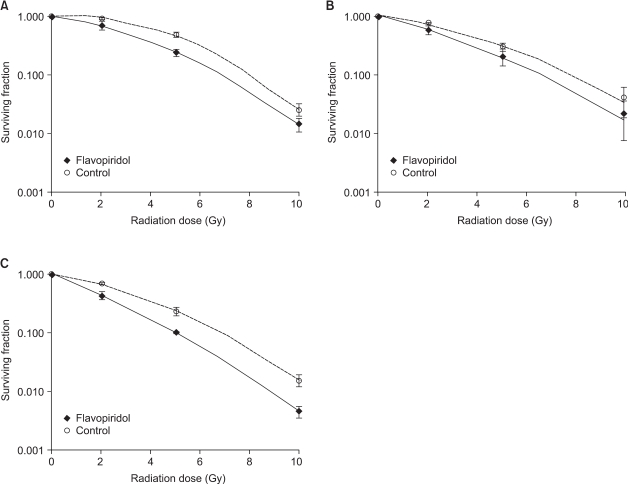
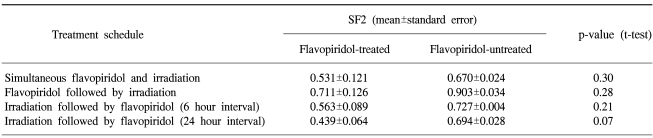
The cell survival curves obtained after the sequential treatments are shown in Fig. 3 (1, 2 & 3). When flavopiridol was administered 24 hours before irradiation, the SF2 values of the flavopiridol-treated and untreated cells were 0.711±0.126 and 0.903±0.034, respectively, which was not a statistically significant difference (p=0.28). When flavopiridol was administered 6 hours after irradiation, the difference between the SF2 values of the flavopiridol-treated and untreated cells was also not statistically significant (0.563±0.089 vs. 0.727±0.004, p=0.21). Flavopiridol resulted in no appreciable enhancement of the radiation effects in the context of these protocols. However, a minor radiosensitizing effect was observed when flavopiridol was administered 24 hours after irradiation. The SF2 values of the flavopiridol-treated and untreated cells were 0.439±0.064 and 0.694±0.040, respectively, which was a marginally significant difference (p=0.07). The SF2 values of the flavopiridol-treated and untreated cells after different treatment protocols are summarized in Table 1.
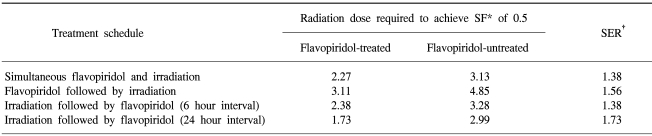
The radiation doses required to achieve an SF value of 0.5 were estimated for flavopiridol-treated and untreated cells, and the SER calculated (Table 2). When comparing the SER values of the four different treatment schedules, we found the SER values to be highest in the cells treated with flavopiridol following irradiation with a 24-hour interval separating the two treatments.
The cytotoxic effects of flavopiridol have been established in a variety of preclinical studies, using many different cancer cell lines, including prostate, breast, colon, lung carcinoma, glioma, squamous cell carcinoma of the head and neck, and lymphoma (8,10~12,14,20). However, the feasibility of the administration of flavopiridol in the treatment of uterine cervix cancer remains to be assessed. In this study, flavopiridol was demonstrated to exert a dose-dependent cytotoxicity on HeLa uterine cervix cancer cells. The IC50 value associated with this treatment, 80 nM, was consistent with those reported in trials with other cell types (6,21,22), and similar to the average (66 nM) reported as a result of the NCI 60 cell line screening.
Flavopiridol has also been reported to augment the cytotoxic actions of several chemotherapeutic agents. Motwani et al. observed that the cytotoxicity of paclitaxel treatment against breast and gastric cancer cell lines was potentiated when flavopiridol was administered after the paclitaxel (17). Pretreatment or concurrent treatment with flavopiridol was found to antagonize paclitaxel-induced apoptosis. Similarly, flavopiridol caused an increase in CPT-11 (SN38)-induced apoptosis in HCT-116 human colon cancer cells, as a result of following the CPT-11 treatment with flavopiridol administration (23).
The present study demonstrated that flavopiridol enhanced the radioresponses of the cervix cancer cell line tested. The most obvious radiosensitizing effect was observed when flavopiridol was administered 24 hours after irradiation. This sequence-dependence was consistent with the results of other trials utilizing gastric and colon cancer cell lines (19). However, the degree of radioenhancement was discovered to be sequence-independent in murine ovarian carcinoma OCA-1 cells (24).
In our study, flavopiridol exerted no appreciable influence on radiation sensitivity when administered 6 hours after irradiation. Kim et al. investigated the time and sequence-related effects of a combination therapy involving docetaxel, flavopiridol and irradiation in H460 human lung cancer cells (25). The interaction between radiation and flavopiridol was also found to be sequence-dependent, with the cell death reaching maximal levels when the radiation treatment preceded the flavopiridol treatment by 2 hours.
As little data currently exists regarding the combination therapy involving flavopiridol and radiation, further study is clearly warranted with regard to the sequence and time-related effects of combination therapies, as well as the mechanisms underlying their actions.
Flavopiridol has never been used by Korean oncologists as an anticancer agent. This in vitro study demonstrates that the drug has the potential for use as a radiosensitizer. More in vivo studies, as well as studies probing the mechanisms underlying its action, will be required before this agent can be used in clinical studies.
Flavopiridol exerted a dose-dependent cytotoxicity on HeLa uterine cervix cancer cells. When combined with radiation, flavopiridol enhanced the effects of radiation on the cells. The most obvious radiosensitizing effect was observed when flavopiridol was administered 24 hours after irradiation.
References
1. MacLachlan TK, Sang N, Giordano A. Cyclins, cyclin-dependent kinases and cdk inhibitors: implications in cell cycle control and cancer. Crit Rev Eukaryot Gene Expr. 1995; 5:127–156. PMID: 8845581.

2. Ahn JG, Lee TS, Cho JW, Baek WK, Suh SI, Suh MH, et al. Expression of cell cycle control genes in human uterine cervical cancer cells. J Korean Cancer Assoc. 2000; 32:110–119.
3. Kelland LR. Flavopiridol, the first cyclin-dependent kinase inhibitor to enter the clinic: current status. Expert Opin Investig Drugs. 2000; 9:2903–2911.

4. Worland PJ, Kaur G, Stetler-Stevenson M, Sebers S, Sartor O, Sausville EA. Alteration of the phosphorylation stte of p34cdc2 kinase by flavone L86-8275 in breast carcinoma cell. Correlation with decreased H1 kinase activity. Biochem Pharmacol. 1993; 46:1831–1840. PMID: 8250970.
5. Losiewicz MD, Carlson BA, Kaur G, Sausville EA, Worland PJ. Potent inhibition of CDC2 kinase activity by the flavonoid L86-8275. Biochem Biophys Res Commun. 1994; 201:589–595. PMID: 8002990.

6. Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996; 56:2973–2978. PMID: 8674031.
7. Meijer L, Kim SH. Chemical inhibitors of cyclin-dependent kinases. Methods Enzymol. 1997; 283:113–128. PMID: 9251015.

8. Kaur G, Stetler-Stevenson M, Sebers S, Worland P, Sedlacek H, Myers C, et al. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J Natl Cancer Inst. 1992; 84:1736–1740. PMID: 1279187.

9. Blagosklonny MV, Darzynkeiwicz Z, Figg WD. Flavopiridol inversely affects p21(WAF1/CIP1) and p53 protects p21-sensitive cells from paclitaxel. Cancer Biol Ther. 2002; 1:420–425. PMID: 12432259.
10. Shapiro GI, Koestner DA, Matranga CB, Rollins BJ. Flavopiridol induces cell cycle arrest and p53-independent apoptosis in non-small cell lung cancer cell lines. Clin Cancer Res. 1999; 5:2925–2938. PMID: 10537362.
11. Alonso M, Tamasdan C, Miller DC, Newcomb EW. Flavopiridol induces apoptosis in glioma cell lines independent of retinoblastoma and p53 tumor suppressor pathway alterations by a caspase-independent pathway. Mol Cancer Ther. 2003; 2:139–150. PMID: 12589031.
12. Newcomb EW, Tamasdan C, Entzminger Y, Alonso J, Friedlander D, Crisan D, et al. Flavopiridol induces mitochondrial-mediated apoptosis in murine glioma GL261 cells via release of cytochrome c and apoptosis inducing factor. Cell Cycle. 2003; 2:243–250. PMID: 12734434.

13. Takaya Y, Aggrawal BB. Flavopiridol inhibits NF-κB activation induced by various carcinogens and inflammatory agents through inhibition of IκBα and p65 phosphorylation: abrogation of cyclin D1, cyclooxygenase-2 and matrix metalloprotease-9. J Biol Chem. 2004; 279:4750–4759. PMID: 14630924.
14. Patel V, Senderowicz AM, Pinto D Jr, Igishi T, Raffeld M, Quintanilla-Martinez L. Flavopiridol, a novel cyclin-dependent kinase inhibitor, suppresses the growth of head and neck squamous cell carcinomas by inducing apoptosis. J Clin Invest. 1998; 102:1674–1681. PMID: 9802881.

15. Melillo G, Sausville EA, Cloud K, Lahusen T, Varsio L, Senderowicz AM. Flavopiridol, a protein kinase inhibitor, down-regulates hypoxic induction of vascular endothelial growth factor expression in human monocytes. Cancer Res. 1999; 59:5433–5437. PMID: 10554012.
16. Bible KC, Kaufmann SH. Cytotoxic synergy between Flavopiridol (NSC 649890, L86-8275) and various antineoplastic agents: the importance of sequence of administration. Cancer Res. 1997; 57:3375–3380. PMID: 9269999.
17. Motwani M, Delohery TM, Schwartz GK. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin Cancer Res. 1999; 5:1876–1883. PMID: 10430095.
18. Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, et al. Suppression of intestinal polyposis in ApcΔ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996; 87:803–809. PMID: 8945508.
19. Jung C, Motwani M, Kortmansky J, Sirotnak FM, She Y, Gonen M, et al. The cyclin-dependent kinase inhibitor flavopiridol potentiates γ-irradiation-induced apoptosis in colon and gastric cancer cells. Clin Cancer Res. 2003; 9:6052–6061. PMID: 14676132.
20. Newcomb EW. Flavopiridol: pleiotropic biological effects enhance its anti-cancer activity. Anticancer Drugs. 2004; 15:411–419. PMID: 15166614.

21. Sedlacek HH, Czech J, Naik R, Kaur G, Worland P, Losiewicz M, et al. Flavopiridol (L86 8275; NSC 649890), a new kinase inhibitor for tumor therapy. Int J Oncol. 1996; 9:1143–1168.

22. Czech J, Hoffmann D, Naik R, Sedlacek HH. Antitumoral activity of flavone L86 8275. Int J Oncol. 1995; 274:1664–1672.
23. Motwani M, Jung C, Sirotnak FM, She Y, Shah MA, Gonen M, et al. Augmentation of apoptosis and tumor regression by flavopiridol in the presence of CPT-11 in HCT-116 colon cancer monolayers and xenografts. Clin Cancer Res. 2001; 7:4209–4219. PMID: 11751522.
24. Mason KA, Hunter NR, Raju U, Ariga H, Husain A, Valdecanas D, et al. Flavopiridol increases therapeutic ratio of radiotherapy by preferentially enhancing tumor radioresponse. Int J Radiat Oncol Biol Phys. 2004; 59:1181–1189. PMID: 15234054.

25. Kim JC, Saha D, Cao Q, Choy H. Enhancement of radiation effects by combined docetaxel and flavopiridol treatment in lung cancer cells. Radiother Oncol. 2004; 71:213–221. PMID: 15110456.

Fig. 1
Cytotoxicity of flavopiridol at nanomolar concentrations. Filled circles represent the experimental data, and the dotted line is a regression line.
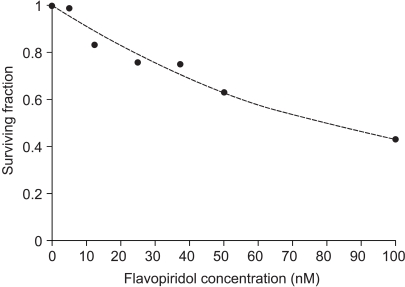
Fig. 2
Surviving fraction of HeLa cells after simultaneous flavopiridol and irradiation treatment, compared with that of cells treated with irradiation alone. Error bars represent standard errors.
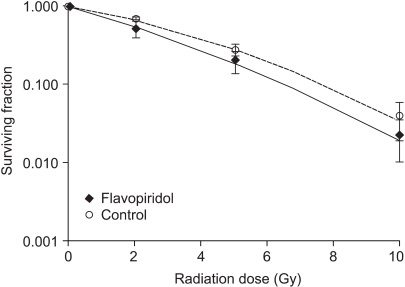
Fig. 3
Surviving fraction of HeLa cells after sequential treatment with flavopiridol and irradiation compared with that of cells treated with irradiation alone. (A) Flavopiridol followed by irradiation after a 24-hour interval, (B) Irradiation followed by flavopiridol after a 6-hour interval, (C) Irradiation followed by flavopiridol after a 24-hour interval. Error bars represent standard errors.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download