Abstract
Purpose
Previous epidemiologic studies have demonstrated that nonsteroidal anti-inflammatory drugs can reduce the risk of breast cancer, and this possibly happens via cyclooxygenase (COX) inhibition. Moreover, growth factor-inducible COX-2, which is overexpressed in neoplastic tissue, is an attractive therapeutic target. Thus, we evaluated the expression of COX-2 in breast cancer tissues, and we assessed the association between COX-2 expression and HER-2/neu expression and also with several clinicopathological features.
Materials and Methods
We analyzed the surgical specimens from 112 women with breast cancer who had undergone lumpectomy or mastectomy. The expressions of COX-2, HER-2/neu, MMP-2 and TIMP-2 were determined immunohistochemically. The correlations between COX-2 expression and several variables, including clinicopathological factors, HER-2/neu expression, MMP-2 expression and TIMP-2 expression were analyzed. Survival analysis was also performed with respect to COX-2 overexpression.
Results
The overexpression of COX-2 protein was observed in 28.6% of the breast cancer tissues. Tumors with lymph node metastasis more frequently showed COX-2 overexpression than did those tumors without metastasis (p=0.039), and the increased COX-2 expression correlated positively with HER-2/neu overexpression (p=0.000). No significant differences were found for the MMP-2 or TIMP-2 expression rates in the COX-2 positive and negative groups. The survival analysis revealed no significant differences according to the COX-2 expression.
Conclusion
This study results suggest that increased COX-2 expression is related with the progression of breast cancer, e.g., with lymph node invasion. COX-2 overexpression found to be related with HER-2/neu overexpression, but not with MMP-2 or TIMP-2 expression. These results support the potential use of selective agents that inhibit COX-2 or HER-2/neu for the management of breast cancer.
Various epidemiologic studies have indicated that the use of aspirin or of other nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with a reduced risk of colon cancer (1), and it may reduce the risk of breast cancer (2). The best-known target of NSAIDs is cyclooxygenase (COX), which is a rate-limiting enzyme that catalyzes the conversion of arachidonic acid to prostaglandins (PGs) and the related eicosanoids. Two isoforms of COX exist in the mammalian body, and the first is COX-1, which is constitutively expressed in most tissues, and it appears to be responsible for the homeostasis of various physiologic functions. In contrast, COX-2 is not detectable in most normal tissues, but it can be induced by cytokines, growth factors, oncogenes and tumor promoters. Moreover, it has a role that's been connected to inflammation and carcinogenesis (3).
The increased expression of COX-2, but not of COX-1, has been found in a variety of human malignancies including colon, lung and breast cancer (4~9). Recent studies have indicated that the overexpression of COX-2 was sufficient to induce breast tumor in a transgenic mouse model (10), and it was also found that increased COX-2 expression and activity is positively correlated with the metastatic potential in a murine model of metastatic breast cancer (11). In addition, the tumor-suppressing effects of selective COX-2 inhibitors on mammary carcinogenesis and metastasis have been reported on animal models of breast cancer (12,13).
Although the mechanisms underlying the contribution of COX-2 to carcinogenesis are unclear, COX-2 and its metabolic products have been shown to inhibit apoptosis (14), promote angiogenesis (15), induce invasion, and increase metastasis (16,17). Matrix metalloproteinases (MMPs) are known to play a central role in tumor cell invasion and metastasis, and several previous reports have mentioned that the overexpression of COX-2 in cancer cells can lead to the activation of MMP-2 (16,17).
The HER-2/neu (c-erbB-2) gene encodes a 185-kDa transmembrane receptor that has tyrosine kinase activity, and it belongs to the epidermal growth factor receptor family. The overexpression of HER-2/neu has been associated with a poor prognosis (18), and recent studies have suggested a link between HER-2/neu and COX-2 expression (19~21).
The aims of this study were to evaluate whether COX-2 expression is increased in human breast cancer tissues and also to investigate the possible association between COX-2 expression and clinicopathological variables, invasion-related parameters and HER-2/neu overexpression.
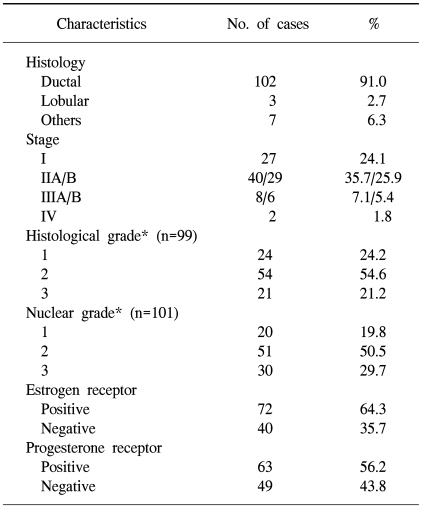
We analyzed the surgical specimens from 112 women with breast cancer who had undergone lumpectomy or mastectomy at Ewha Womans University Hospital in Seoul, Korea from 1993 to 1998. These patients' hospital records were reviewed retrospectively. The surgical specimens that were obtained by lumpectomy or mastectomy were formalin fixed, paraffin embedded, sectioned and then stained with hematoxylin and eosin. The pathological tumor staging was determined according to the TNM classification (22). The histological and nuclear grading was assessed according to the modified Bloom-Richardson criteria (23) in the cases with invasive ductal carcinoma. Table 1 presents the patients' data and the various clinicopathological characteristics.
The patients' age at the time of diagnosis ranged from 23 to 76 years (median age, 46 years), and 36 (32%) women were over 50 years of age. Sixty-six percent of the patients received-adjuvant chemotherapy, 79% of the patients received antiestrogen therapy and 33% of the patients received adjuvant radiotherapy. The median follow-up duration was 106 months, and during this time, 22 cases (20%) had relapsed.
The routinely fixed paraffin-embedded blocks were sectioned at 4 µm thickness and then processed for immunohistochemistry. Sections were deparaffinized, rehydrated and microwaved in 0.1M citrate buffer for 10 min for antigen retrieval. After washing, slides were incubated in 3% H2O2 for 8 min to block the endogenous peroxidase activity, and next they were immunostained. All the immunostaining was performed using a Novostain Universal Quick Kit (Novocastra, New Castle upon Tyne, UK). Briefly, the slides were preincubated with normal horse serum for 20 min, and then they were incubated with anti-COX-2 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, 1:200 dilution), anti-HER-2/neu monoclonal antibody (Novocastra, 1:100 dilution), anti-MMP-2 monoclonal antibody (Oncogene Research Products, Cambridge, MA, 1:100 dilution), and anti-TIMP-2 monoclonal antibody (Neo Markers, Fremont, CA, 1:50 dilution) for 40 min at room temperature. The slides were then washed in Tris buffer; biotinylated secondary antibody was next applied for 25 min at room temperature, and the slides were washed in Tris buffer. Streptavidin peroxidase complex (Novostain universal quick kit, Novocastra) was applied for 25 min at room temperature, and the reaction products were visualized using AEC (3-amino-9-ethylcarbazole, Zymed, San Francisco, CA), and then they were counterstained with hematoxylin.
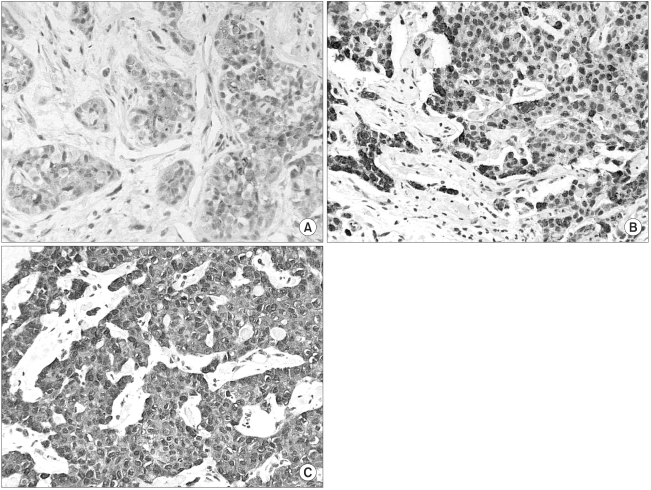
Semi-quantitative estimations were performed based on the staining intensity and the relative abundance of immunoreactive cells. For COX-2, the scoring criteria for tumor cells was as follows: 0, fewer than 10% positively stained tumor cells; 1, 11~30% positively stained tumor cells or weak diffuse cytoplasmic staining; 2, moderate to strong cytoplasmic staining in 31~60% of the tumor cells; 3, 61~100% of tumor cells were stained with a strong intensity (Fig. 1). For the HER-2/neu expression, the same cut-off points for positive tumor cells were used, but the positive tumor cells were accounted for by membranous staining only.
For MMP-2 and TIMP-2, the scoring criteria for the tumor cells were as follows: 0, ≤10% positively stained tumor cells; 1, 11~30% positively stained tumor cells or there was weak diffuse cytoplasmic staining; 2, moderate to strong cytoplasmic staining in more than 30% of the tumor cells.
The overexpression of COX-2 (the COX-2 positive group) and HER-2/neu (the HER-2 positive group) were defined as a score of 3. Scores of 1 and 2 were allocated for the overexpression of MMP-2 and TIMP-2.
The statistical analysis was carried out using SPSS version 11.0 for Windows. The χ2-test was used to test for correlations between the COX-2 expression and the clinicopathological variables, the HER-2/neu expression, the MMP-2 expression or TIMP-2 expression. The overall survival and disease-free survival were calculated using the Kaplan-Meier method. Disease-free survival was defined as the time from the date of the operation until a documented recurrence or until death from any cause. The overall survival was measured from the date of the diagnosis. Survival curves for the positive and negative COX-2 and HER-2/neu groups were compared by using the log-rank test. A p value of < 0.05 was considered statistically significant.
COX-2 immunoreactivity was evaluated in 112 invasive breast carcinomas, of which 3 cases (2.7%) were scored 0, 21 cases (18.7%) were scored 1, 56 cases (50%) were scored 2, and 32 cases (28.6%) were scored 3. The overexpression of COX-2 protein (the COX-2 positive group) was observed in 28.6% of the tumors. The COX-2 staining was granular and localized to the tumor cytoplasm (Fig. 1), and this was also observed in the adjacent ductal carcinoma in situ (DCIS) lesions.
Overexpression of HER-2/neu (the HER-2/neu positive group) was observed in 42.9% (48 of 112 cases) of the tumors. The MMP-2 and TIMP-2 immunoreactivity were detected in 68.2% (75 of 110 cases) and 62.2% (69 of 111 cases) of the cases in the cytoplasm of the tumor cells. The expressions of MMP-2 and TIMP-2 were also observed in the tumor stroma.
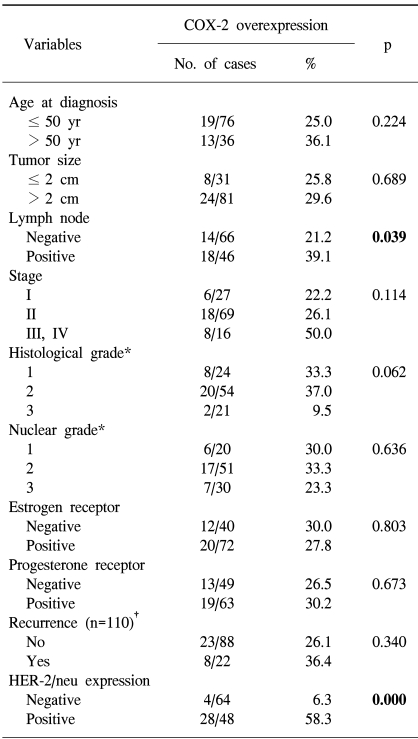
The overexpression of COX-2 was significantly more frequent in the HER-2/neu overexpression group (58.3%) than in the HER-2/neu negative group (6.3%) (p=0.000; Table 2). An elevated COX-2 expression was more common in tumors with axillary lymph node metastasis (39.1%) than in those tumors without lymph node metastasis (21.2%) (p=0.039). According to the stage, the COX-2 overexpression rate in the advanced stage tumors (50.0% in the stage III and IV tumors) was higher than in the early stage tumors (22.2% in stage I tumors and 26.1% in stage II tumors), but this was not significant (p=0.114). No significant correlation was found between the COX-2 overexpression and the other clinicopathological variables (Table 2).
MMP-2 tumor expression was observed in 71.9% of the COX-2 positive group and in 66.7% of the COX-2 negative group, but the difference was not significant (p=0.594). No significant difference in the TIMP-2 expression rate was found between the COX-2 positive group (62.5%) and the COX-2 negative group (62%) (p=0.963).
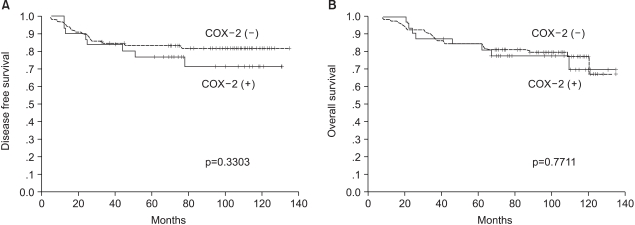
The 8-year disease-free survival rates for the COX-2 positive and negative groups were 71.2% and 82.0% (p=0.3303), respectively, and the 8-year overall survival rates of these two groups were also similar (77.8% vs 79.7%, respectively, p=0.7711) (Fig. 2).
In terms of HER-2/neu overexpression, the 8-year disease-free survival rate of the HER-2/neu positive group was 67.8% compared with 87.2% for the HER-2/neu negative group (p=0.0290). However, there was no significant difference for the 8-year overall survival rates between the HER-2/neu positive group and the HER-2/neu negative group (71.7% vs 84.3%; respectively, p=0.2423).
We detected the overexpression of COX-2 protein in 28.6% of the human breast cancers we examined, as compared to 17.4% to 77.8% of the breast cancer tissues that were examined in several previous studies (4~9). The suggested causes for this diversity of the COX-2 overexpression rate were that different cut-off values were used in all the studies and there were different populations with different patient characteristics in each study.
COX-2 overexpression was restricted to the tumor cells, whereas the stroma showed as being either COX-2 negative or weakly positive. In addition, COX-2 protein was expressed in the DCIS as well as in invasive breast cancer. These results are in accordance with the previous data (4,7), and they suggest that COX-2 overexpression is a relatively early phenomenon in mammary tumorigenesis. However, most of the DCIS lesions, included in these previous studies, were adjacent to the invasive tumors, and a further evaluation of COX-2 in patients with only DCIS is warranted.
In this study, the COX-2 expression rate was significantly higher in the HER-2/neu overexpression group than in the HER-2/neu negative group (58.3% vs 6.3%, respectively). HER-2/neu is known to be a poor prognostic factor in breast cancer, and its relationship to the regulation of COX-2 expression has been suggested in recent studies (19~21). In human colorectal cancer cells, the activation of the HER-2 pathway induced COX-2 mRNA and protein as well as PGE2 biosynthesis (19). In addition, HER-2/neu stimulated COX-2 transcription via the Ras pathway in human mammary epithelial cells (20). Similarly, in human breast cancer tissue, the relationship between HER-2/neu and COX-2 expression has been revealed (6,9). Half et al. (7) did not find an association between COX-2 and HER-2/neu in the breast cancer tissues that they studied by using immunohistochemistry, but they reported that COX-2 expression was induced in the MCF-7 cells that were stably transfected with HER-2/neu, and this was in contrast to the COX-2 expression observed in the parental MCF-7 cells. Furthermore, the antineoplastic effects of selective COX-2 inhibitors on HER-2/neu-induced breast tumor have been reported (21,24). In the mouse mammary tumor virus (MMTV)/neu model (21), a selective COX-2 inhibitor significantly reduced the incidence of HER-2/neu-induced breast tumor. In HCA-7 colorectal carcinoma cells (24), the combined use of COX-2 and HER-2/neu inhibitors more effectively reduced the colorectal carcinoma growth than using either of the inhibiting agent alone. These results suggest that a correlation may exist between the regulation of the COX-2 and HER-2/neu pathways, and that the specific inhibitors of both of these pathways can be exploited for their use in experimental breast cancer chemotherapeutic trials.
Our results showed that COX-2 overexpression was related with the presence of axillary lymph node metastasis. However, no correlation was found between COX-2 overexpression and the other clinicopathological prognostic factors, and no differences were observed in the MMP-2 and TIMP-2 expression rates with respect to COX-2 expression. Diverse results have been reported concerning the relationship between COX-2 expression and the clinicopathological characteristics of tumors (5~9). Although some of these studies found no relationship (5,7,9), Ristimäki et al. (6) found that COX-2 positivity was correlated with several parameters such as large tumor size, the presence of axillary node metastases, a high histological grade and a negative hormone receptor status in human breast cancer. Moreover, Costa et al. (8) showed that COX-2 expression was significantly associated with lymph node metastasis in human breast cancer. In a murine model of breast cancer (11), COX-2 expression was detected only in the tumors that resulted from the transplantation of metastatic mammary tumor cell lines in vivo. These results suggest a linkage between COX-2 expression, tumor progression and the metastatic potential. However, the underlying mechanisms for the contributions made by COX-2 to tumor progression and metastasis remain somewhat obscure. The expression and activation of the MMPs that are related to the process of cancer invasion and metastasis were observed in human colon and breast cancer cells that were transfected with COX-2 cDNA (16,17). On the other hand, we found no association between MMP-2, TIMP-2 expression and COX-2 positivity in human breast cancer tissues. Thus, further studies are needed to determine the role of COX-2 in tumor progression and metastasis.
The prognostic significance of COX-2 expression on the survival of breast cancer patients is still controversial (6,8,9). Ristimaki et al. (6) have demonstrated that elevated levels of COX-2 expression are associated with decreased survival for patients with breast cancer, and that these survival differences tend to be more marked for the patients in the ER positive, p53 negative and HER-2 negative cancer subgroups. They suggested that the procarcinogenic effect of COX-2 was not evenly distributed in breast cancer. In contrast, we do not find the prognostic role of COX-2 overexpression, but our results did show a lower disease free survival rate in the HER-2/neu overexpression group (p=0.0290). The HER-2/neu overexpression rate, as tested for by immunochemistry, varies in the wide range from 9% to 60% (25). The high rate of HER-2/neu overexpression seen in this study was affected by the immunohistochemical method and the particular patient population that we used.
We suggest that the limitations of this study were the small number of patients, the retrospective study design and the different postoperative management strategies that were used, and we propose that large scale, prospective studies are needed to define the definitive prognostic role of COX-2.
Our study indicates that COX-2 overexpression is related to the progression of breast cancer such as to the lymph node metastasis, and to HER-2/neu overexpression. These results support the therapeutic potential of using selective agents that inhibit COX-2 and/or HER-2/neu in the management of breast cancer. Further studies are needed to determine the effects of selective COX-2 inhibitors for the prevention and adjuvant therapy of breast cancer.
References
1. Thun MJ, Namboodiri MM, Heath CW Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991; 325:1593–1596. PMID: 1669840.

2. Khuder SA, Mutgi AB. Breast cancer and NSAID use: a meta-analysis. Br J Cancer. 2001; 84:1188–1192. PMID: 11336469.

3. Dempke W, Rie C, Grothey A, Schmoll HJ. Cyclooxygenase-2: a novel target for cancer chemotherapy? J Cancer Res Clin Oncol. 2001; 127:411–417. PMID: 11469677.

4. Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000; 89:2637–2645. PMID: 11135226.

5. Kang HJ, Gong G, Jang SJ, Jung PJ, Park CK. Expression of cyclooxygenase-2 in human breast carcinoma: relevance to tumor angiogenesis and expression of estrogen receptor. Cancer Res Treat. 2001; 33:286–295.

6. Ristimäki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002; 62:632–635. PMID: 11830510.
7. Half E, Tang XM, Gwyn K, Sahin A, Wathen K, Sinicrope FA. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002; 62:1676–1681. PMID: 11912139.
8. Costa C, Soares R, Reis-Filho JS, Leitao D, Amendoeira I, Schmitt FC. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol. 2002; 55:429–434. PMID: 12037025.

9. Ahn JH, Kim SB, Ahn SH, Gong GY, Ahn MJ, Kang YK, et al. Clinical value of cyclooxygenase-2 expression in human breast carcinoma. Cancer Res Treat. 2004; 36:192–198.

10. Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001; 276:18563–18569. PMID: 11278747.

11. Kundu N, Yang Q, Dorsey R, Fulton AM. Increased cyclooxygenase-2 (COX-2) expression and activity in a murine model of metastatic breast cancer. Int J Cancer. 2001; 93:681–686. PMID: 11477578.

12. Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000; 60:2101–2103. PMID: 10786667.
13. Kundu N, Fulton AM. Selective cyclooxygenase (COX)-1 or COX-2 inhibitors control metastatic disease in a murine model of breast cancer. Cancer Res. 2002; 62:2343–2346. PMID: 11956094.
14. Watson AJ. Chemopreventive effects of NSAIDs against colorectal cancer: regulation of apoptosis and mitosis by COX-1 and COX-2. Histol Histopathol. 1998; 13:591–597. PMID: 9589912.
15. Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000; 60:1306–1311. PMID: 10728691.
16. Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997; 94:3336–3340. PMID: 9096394.

17. Takahashi Y, Kawahara F, Noguchi M, Miwa K, Sato H, Seiki M, et al. Activation of matrix metalloproteinase-2 in human breast cancer cells overexpressing cyclooxygenase-1 or -2. FEBS Lett. 1999; 460:145–148. PMID: 10571077.

18. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987; 235:177–182. PMID: 3798106.
19. Vadlamudi R, Mandal M, Adam L, Steinbach G, Mendelson J, Kumar R. Regulation of cyclooxygenase-2 pathway by HER2 receptor. Oncogene. 1999; 18:305–314. PMID: 9927187.

20. Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem. 2002; 277:18649–18657. PMID: 11901151.
21. Howe LR, Subbaramaiah K, Patel J, Masferrer JL, Deora A, Hudis C, et al. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 2002; 62:5405–5407. PMID: 12359744.
22. American Joint Committee on Cancer. AJCC cancer staging manual. 1997. 5th ed. Philadelphia: Lippincott-Raven;p. 171–180.
23. Elston CW, Ellis IO. Elston CW, Ellis IO, editors. Assessment of histological grade. The breast: Systemic pathology. 1998. Vol 13:3rd ed. Edinburgh: Churchill Livingstone;p. 368–376.
24. Mann M, Sheng H, Shao J, Williams CS, Pisacane PI, Sliwkowski MX, et al. Targeting cyclooxygenase 2 and HER-2/neu pathways inhibits colorectal carcinoma growth. Gastroenterology. 2001; 120:1713–1719. PMID: 11375952.

25. Lebeau A, Deimling D, Kaltz C, Sendelhofert A, Iff A, Luthardt B, et al. Her-2/neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol. 2001; 19:354–363. PMID: 11208826.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download