Abstract
A 13 year-old girl with osteosarcoma and pulmonary tumor recurrence developed acute renal failure following high dose methotrexate (12 g/m2) therapy, she had previously tolerated high dose methotrexate and her renal and hepatic functions were normal. Briefly, 48 hours after beginning methotrexate infusion her methotrexate concentration and creatinine level were 1338.8 µM/L and 5.8 mg/dl, respectively. Grade IV oral mucositis and neutropenia with fever developed at 144 hours after MTX infusion. Hydration and alkalinization were continued and leucovorin rescue was intensified based on the plasma MTX concentrations. Plasma exchange was performed twice and hemodialysis 3 times without problems, but methotraxate and creatinine levels remained high, 91.9 µM/L, and 2.5 mg/dl, respectively. After 3 courses of hemodialysis carboxypeptidase-G2 (CPDG2) was administered at 50 U/kg, intravenously over 5 minutes. After 15 minutes of CPDG2 (Voraxaze™) infusion, her plasma MTX concentration was 0.91 µM/L and no rebound elevation or side effects developed. Thirteen days post-MTX infusion her renal function had normalized. We report here our experience of a dramatic methotrexate level reduction caused by CPDG2 administration.
Methotrexate (MTX), a classic antifolate, is one of the most widely used and well studied anticancer agents however high-dose methotrexate (HDMTX) induced renal failure presents a medical emergency situation, as MTX is primarily eliminated by renal excretion (1). Delayed MTX excretion and sustained elevated plasma MTX concentrations may markedly enhance other MTX toxicities for example, myelosuppression, orointestinal mucositis, hepatitis, and dermatitis.
Because there is no centralized reporting system for MTX nephrotoxicity, the current incidence of HDMTX-induced renal dysfunction is unknown. Conventional treatment for HDMTX-induced renal dysfunction includes a prompt increase in the leucovorin (LV) dose based on plasma MTX concentration (2,3) and the continued hydration and alkalinization, providing an adequate urine output can be maintained. High doses of LV do not necessarily prevent toxicity in the presence of a sustained elevated plasma MTX concentration. Hemodialysis-based methods of MTX removal have been described over the past 20 years (4,5). More recently, carboxypeptidase-G2 (CPDG2) (6), a recombinant bacterial enzyme that hydrolyzes MTX to the inactive metabolite 2, 4-diamino-N10-methylpteroic acid (DAMPA) (7), has become available as an investigational new drug for the treatment of HDMTX-induced renal dysfunction. Here we report our experience of the use of CPDG2 in a patient with osteosarcoma.
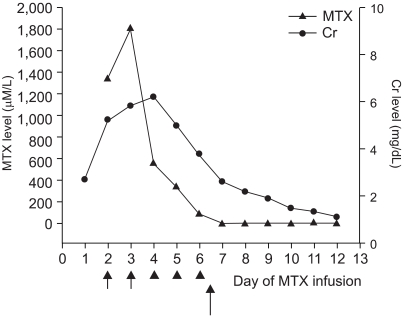
A 13 year-old girl with osteosarcoma of the right humerus underwent neoadjuvant chemotherapy based on the CCG 7921A regimen (8) (includes cisplatin, adriamycin, and high dose methotrexate), wide excision, and prosthesis insertion. Eleven months after her chemotherapy, she presented with pulmonary tumor recurrence, and was treated with ifosfamide, etoposide, and high dose methotrexate (12 g/m2). Methotrexate had been used previously without problem. Prechemotherapy renal and hepatic functions were within normal limits. After hydration and alkalinization of urine, she received MTX (total 17 g) as a 4-hour infusion and intravenous LV rescue was initiated 24 hour after beginning MTX infusion at 15 mg/m2 every 6 hr. Forty-eight hours post-MTX infusion, her creatinine and blood urea nitrogen levels were 5.0 mg/dl and 40 mg/dl, respectively. Because her plasma MTX concentration at 48 hours was 1338.8 µM/L, plasma exchange was initiated using a Cobe spectra (Gambro BCT, Inc., Lakewood, COL) via a double-lumen femoral dialysis catheter. Leucovorin rescue was continued based on monitored plasma MTX concentrations. After two plasma exchange, her plasma MTX concentration was 553.0 µM/L, but creatinine and blood urea nitrogen levels were 6.2 mg/dl and 73 mg/dl, respectively. Because her creatinine level remained high, and despite plasma exchange, hemodialysis were performed for 3 days without any complications. However grade IV oral mucositis and neutropenia with fever developed at 144 hours after MTX infusion. Antibiotics and antifungal agents, and GM-CSF (250 µg/m2) were administered. Total parenteral nutrition was needed. After 2 consecutive hemodialyses, her plasma MTX concentration was 91.9 µM/L, and her creatinine and blood urea nitrogen levels were 3.8 mg/dl and 35 mg/dl, respectively. Then after receiving her parents' written informed consent, CPDG2 (Voraxaze™, reconstitution of Voraxaze™ in normal saline, 50 U/kg) was administered intravenously over 5 minutes. She had no complaint during or after the Voraxaze™ infusion and blood samples were obtained immediately before and 15, 30, 45, and 60 minutes after Voraxaze™ infusion and then once daily. Blood samples were then placed on ice during transportation to the laboratory. Plasma MTX concentrations were 91.9, 0.91, 0.92, 0.94, and 0.95 µM/L at basal, and at 15, 30, 45, and 60 minutes after Voraxaze™ infusion, respectively as determined by enzyme-linked immunosorbent assay (ELISA) (Fig. 1). Thirteen days post-MTX infusion renal function normalized and she was discharged 2 days later without problem.
The median incidence of reported renal toxicity due to HDMTX in individual studies is ca. 1.5% (range, 0.0~12.4%). There were 3,887 patients enrolled on osteosarcoma trials in which renal toxicity data were available (9). Sixty-eight (1.8%) of these 3,887 patients developed nephrotoxicity that was either grade 2 or significant enough to be reported, and 23 patients (0.6%) developed grade 3 or 4 toxicity. Three deaths were attrubutable to HDMTX-induced renal dysfunction among 68 patients, giving a 4.4% mortality rate (2).
Sohn et al (10) reported upon the efficacy of pre- and post-operative chemotherapy in patients with osteosarcoma of the extremities but found no grade 3 or 4 nephrotoxicity after HDMTX. Kim et al (11) reported the HDMTX induced nephrotoxicity in case of malignant lymphoma; this was managed by plasma exchange and hemodialysis to reduce the MTX toxicity. However, this is the first report about CPDG2 rescue in a patient with HDMTX-induced nephrotoxicity in Korea.
The use of dialysis-based method for MTX removal resulted in a median decrease in plasma MTX concentrations of 52% (range, 26~82%). Dialysis-based methods were used for up to 14 days. However, rebound increases in post-dialysis plasma MTX concentrations of 10~221% of post-dialysis MTX levels (12) and of 90~100% of the pre-dialysis MTX levels have been reported (13). However, CPDG2 administration resulted in a rapid 95.6~99.6% reduction (median, 98.7%) in plasma MTX concentrations, as measured by high-pressure liquid chromatography due to the interference of MTX metabolites with commercially available immunoassays. Plasma MTX concentrations were found to reduce from median levels of 89 µM/L and 6.4 µM/L to 1 µM/L and <0.05 µM/L within 15 minutes of CPDG2 administration in patients with osteosarcoma and non-Hodgkin lymphoma or acute lymphoblastic leukemia, respectively (14). A rebound increase in plasma MTX concentrations was observed in 60% of patients who were treated with CPDG2, which amounted to 2.8% of the MTX concentration prior to CPDG2 administration in patients with osteosarcoma (14). In our patient the plasma MTX concentration decreased by 99.02% after 15 minutes of CPDG2 administration (from 91.9 µM/L to 0.91 µM/L) and no rebound increase was detected. Our patient had no side reaction. However, 4 of 20 patients described mild symptoms associated with CPDG2 administration, these included a feeling of warmth, flushing, tingling fingers, head pressure, shaking, and burning of face and extremities (14).
One theoretical concern regarding the use of CPDG2 involves the rapid formation of the MTX metabolite, DAMPA, which is approximately 10-fold less soluble than MTX. The potential benefits of the administration of CPDG2 over the use of dialysis-based methods include the more rapid and profound lowering of plasma MTX concentrations, its ease of use (single intravenous infusion) and the lack of a need to insert a new vascular-access device and therefore the avoidance of its associated risks. Rebounds of <10% in plasma MTX concentrations were observed in only 60% of patients treated with CPDG2 (14). Therefore, the use of CPDG2 instead of dialysis-based methods is recommended to reduce plasma MTX concentrations in patients with HDMTX-induced renal dysfunction.
ACKNOWLEDGMENTS
We thank Protherics Limited for supplying Voraxaze™ and Mayne Pharma (SEA) Pte Ltd. (Singapore) and DB Pharm Korea Co., Ltd making transport arrangements.
References
1. Lankelma J, van der Klein E, Ramaekers F. The role of 7-hydroxymethotrexate during methotrexate anti-cancer therapy. Cancer Lett. 1980; 9:133–142. PMID: 7189691.

2. Bleyer WA. Methotrexate: clinical pharmacology, current status and therapeutic guidelines. Cancer Treat Rev. 1977; 4:87–101. PMID: 329989.

3. Flombaum CD, Meyers PA. High-dose leucovorin as sole therapy for methotrexate toxicity. J Clin Oncol. 1999; 17:1589–1594. PMID: 10334548.

4. Relling MV, Stapleton FB, Ochs J, Jones DP, Meyer W, Wainer IW, et al. Removal of methotrexate, leucovorin, and their metabolites by combined hemodialysis and hemoperfusion. Cancer. 1988; 62:884–888. PMID: 3261621.

5. Kawabata K, Makino H, Nagake Y, Tokioka H, Matsumi M, Morita Y, et al. A case of methotrexate-induced acute renal failure successfully treated with plasma perfusion and sequential hemodialysis. Nephron. 1995; 71:233–234. PMID: 8569964.
6. Minton NP, Atkinson T, Sherwood RF. Molecular cloning of the Pseudomonas carboxypeptidase G2 gene and its expression in Escherichia coli and Pseudomonas putida. J Bacteriol. 1983; 156:1222–1227. PMID: 6358192.
7. Widemann BC, Sung E, Anderson L, Salzer WL, Balis FM, Monitjo KS, et al. Pharmacokinetics and metabolism of the methotrexate metabolite, 2,4-diamino-N10-methylpteroic acid. J Pharmacol Exp Ther. 2000; 294:894–901. PMID: 10945838.
8. Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982; 49:1221–1230. PMID: 6174200.
9. Widemann BC, Balis FM, Kempf-Bielack B, Bielack S, Pratt CB, Ferrari S, et al. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004; 100:2222–2232. PMID: 15139068.

10. Sohn JH, Rha SY, Jeung HC, Shin HJ, Goo YS, Chung HC, et al. Efficacy of pre- and postoperative chemotherapy in patients with osteosarcoma of the extremities. Cancer Res Treat. 2001; 33:520–526.

11. Kim PS, Ko YH, Jung JE, Kim CW, Cho SG, Kim HK, et al. A case of high dose methotrexate-induced acute renal failure successfully treated with plasma exchange and hemodialysis. Korean J Hematol. 2000; 35:58–61.
12. Greil J, Wyss PA, Ludwig K, Bonakdar S, Scharf J, Beck JD, et al. Continuous plasma resin perfusion for detoxification of methotrexate. Eur J Pediatr. 1997; 156:533–536. PMID: 9243235.

13. Jambou P, Levraut J, Favier C, Ichai C, Milano G, Grimaud D. Removal of methotrexate by continuous venovenous hemodiafiltration. Contrib Nephrol. 1995; 116:48–52. PMID: 8529381.

14. Widemann BC, Balis F, Murphy RF, Sorensen JM, Montello MJ, et al. Carboxypeptidase-G2, thymidine, and leucovorin rescue in cancer patients with methotrexate-induced renal dysfunction. J Clin Oncol. 1997; 15:2125–2134. PMID: 9164227.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download