Abstract
Purpose
The purpose of this study was to evaluate the clinicopathological significance of the microvessel density and macrophage and mast cell counts in invasive breast carcinomas.
Materials and Methods
45 invasive breast carcinomas were immunohistochemically stained with the endothelial antigen, CD34, and macrophage marker, CD68. 0.1% toluidine blue was used to highlight mast cells. The microvessel and mast cell counts were performed at ×200 magnification and the macrophages at ×400 magnification.
Results
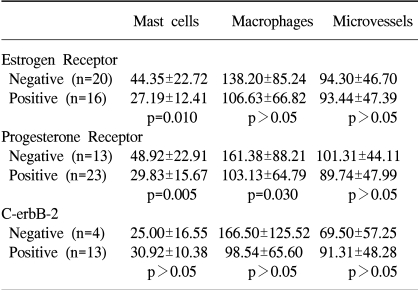
With the 45 invasive breast carcinomas, there were no statistically significant associations between the mast cell, macrophage and microvessel counts and the tumor size and lymph node status. ER and PR negative mast cells infiltrated more than in cases of positive stati, with statistical significance (p-value=0.010 and 0.005, respectively). The macrophage counts were negatively correlated with the PR status (p-value=0.030). With respect to the c-erbB-2 status, there was no significance correlation with the mast cell, macrophage and microvessel counts. The mast cell counts showed significantly positive correlation with the microvessel counts in the invasive breast carcinomas (p-value=0.015). In a comparison of the macrophage counts with the microvessel counts, a positive tendency for both parameters, but without statistical significance (p-value=0.310).
Conclusion
Increasing numbers of mast cells and macrophages were recruited in invasive breast carcinomas, which contribute to angiogenesis. The microvessel density in invasive breast carcinomas had no statistically significant association with the tumor size, lymph node status, and histological grade, presence of DCIS component, estrogen/progesterone receptor status and cerbB-2 status. The evaluation of angiogenesis using these methods is not thought to provide an independent clinicopathological factor in invasive breast carcinomas.
Neoplastic transformation evokes an immune response, which morphologically manifests as peritumoral and intratumoral inflammatory cell infiltrates. Inflammation related host cells, including fibroblasts, macrophages and mast cells, which are recruited and activated by tumor cells, via a paracrine mechanism, act synergistically with cells by secreting the same or other factors (1).
Experimentally induced tumors display mast cell accumulation close to the tumor cells before the onset of angiogenesis (2), and those induced in mast cell-deficient mice showed both reduced angiogenesis and metastatic potential (3).
Macrophages play a role in the immune response via the elaboration of cytokines and growth factors. The presence of macrophages has been reported in various tumors, suggesting that high macrophage counts were associated with unfavorable outcomes in laryngeal carcinomas (4) and meningiomas (5).
Angiogenesis is obligatory in the enhancement of solid tumor progression, such as the growth, invasion and metastasis of solid tumors (6). Endothelial cells also secrete a variety of matrix-degrading proteinases, which facilitate invasion (7).
Many studies on the association between angiogenesis, tumor dissemination and their outcome have been performed. Correlations have been reported between microvessel counts and prognosis of melanomas and carcinomas of the breast, lung, prostate, head and neck, as well as other tumors. In addition, a relation was noted between the macrophage density and angiogenesis in breast cancers in a 42 case report, where the macrophage density was found to be positively correlated with the microvessel density, suggestive of vascular invasion (8). Similarly high macrophage counts were associated with high vessel counts, and reduced relapse-free and overall survivals in invasive breast carcinomas (9).
In this study, we analyzed the relationship between the macrophage and mast cell counts and the microvessel density in 45 invasive breast carcinomas, with a comparison to the available clinicopathological parameters.
The study population was composed of 45 female breast invasive ductal carcinomas, which had been surgically diagnosed at Chung-Ang University hospital between 1999 and 2003. The pathological materials and reports were reviewed to determine the clinicopathological parameters, including tumor size, lymph node status, histological and nuclear grades, the ductal carcinoma in situ (DCIS) component, hormone receptor (progesterone and estrogen) and c-erbB-2 stati.
According to the Nottingham/Tenovus Breast Cancer study's modification of the Bloom and Richardson system, the histological and nuclear grades, with respect to the degree of tubular formation, nuclear pleomorphism, mitosis and nucleolar size, the tumors were graded as grades 1, 2, and 3. The tumor size was classified as T1 (2 cm or less than 2 cm), T2 (more than 2 cm and less than 5 cm) and T3 (more than 5 cm) using the American Joint Committee on Cancer. Metastatic status of lymph node, and were grouped into negative or positive, but without considering the number of metastasized lymph nodes. The presence of DCIS components was histopathologically scanned. The immunohistochemical estrogen and progesterone receptors (ER and PR) and c-erbB-2 stati were reviewed from the biopsy reports.
To evaluate the microvessel density, macrophage counts and mast cells, specimens were stained using the usual immunohistochemical ABC method and toluidine blue. The monoclonal antibodies for endothelial cells and macrophages were CD34 (Santa Cruz, UK) and CD68 (Zymed, San Francisco, CA), respectively.
For the microvessel counts, all available slides were examined at ×40 to identify the 3 areas with the highest number of CD34 positive vessels within the tumors. Counts were carried out at ×200 field, and averaged. Vessels of a caliber larger than approximately 8 red blood cells, with thick muscular walls and sclerotic areas were excluded from the count. Single or clusters of endothelial cells, with or without a lumen, were regarded as individual vessels (10). For the macrophage counts, 3 fields surrounding the tumors, chosen at areas of maximal inflammatory infiltrates, were screened, counts performed and then averaged at high-power magnification. Mast cells highlighted with toluidine blue stain were counted three times at ×200 magnification, and then averaged.
Statistical analyses were performed using the SPSS-PC package (version 10.0 SPSS, Chicago, 2003). Continuous variables were compared using independent t-tests for two groups and one-way ANOVA for three or more groups. Correlations between the microvessel and mast cell and macrophage counts were assessed using Pearson's (r) coefficient and simple regression analysis.
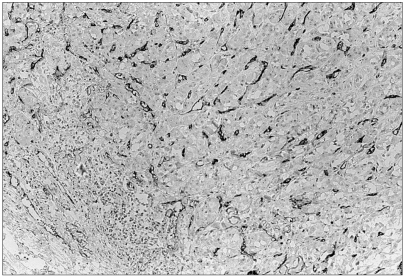
Mast cells were mainly distributed in between expanding tumor nests and non-tumoral interstitial tissues (Fig. 1). Among the 45 invasive breast carcinomas, the mast cell counts ranged from 10 to 98 at ×200 magnification (mean 38.02±21.50). The density of CD68-positive macrophages in the invasive breast carcinomas ranged from 15 to 350 (mean 120.29±78.05) at ×400 magnification. Macrophages were scattered intratumorally, peritumorally and around the tumor necrosis (Fig. 2). CD34 positive microvessels were seen both within and in the vicinity of invasive tumor nests (Fig. 3). The microvessels ranged from 10 to 250 (mean 98.49±49.91) at ×200 magnification.
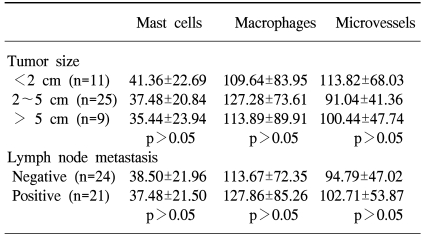
No statistically significant associations were found between the mast cell, macrophage and microvessel counts with respect to the tumor size and lymph node status (Table 1).
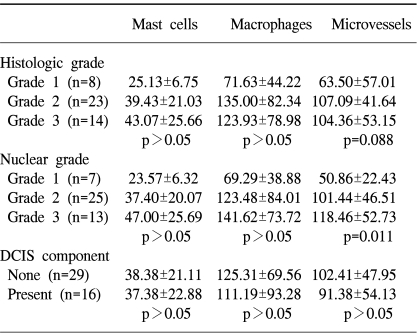
The mast cell counts had a tendency to increase with the higher nuclear and histological grades, but without statistical significance (p-value=0.062). S statistically significant relationship was found between the microvessel density and nuclear grade (p-value=0.011), but had a slight tendency to increase with the histological grade (p-value=0.088). The macrophage counts showed no association with the nuclear and histological grades. With respect to the DCIS component, there was no statistically significant difference between the mast cell, macrophage and microvessel counts (Table 2).
A statistically significant relationship was found between negative ER and PR stati and mast cell infiltration (p-value=0.010 and 0.005, respectively). Macrophage counts had a negative correlation to the PR status (p-value=0.030). There was no statistical significance between the mast cell, macrophage and microvessel counts with respect to the c-erbB-2 status (Table 3).
The mast cell counts showed a positive correlation with those of the microvessels (Pearson's (r) coefficient=0.362, p-value=0.015, Fig. 4). The macrophage counts had a tendency to increase in relation to the microvessel density, but without statistical significance (Pearson's (r) coefficient=0.155, p-value=0.310, Fig. 5). Macrophages and mast cells were positively correlated, which was statistically significant (Pearson's (r) coefficient=0.298, p-value=0.047, Fig. 6).
With the introduction and advancement of different techniques for the management of breast cancer, knowledge of the factors that may influence tumor behavior and the disease course have become increasingly important in assigning patients for different treatments. Several prognostic factors have been identified, and successfully used in a clinical setting. The known factors are stage at presentation, tumor grades, histological types, vascular invasion, and estrogen and progesterone receptor stati. Many additional factors have also been investigated, including the proliferative activity and ploidy status of the tumor, amplification of the HER2/neu oncogene and overexpression of cathepsin-D.
Many studies have reported that suggest tumor angiogenesis, expressed as the number of microvessels within the tumor, is significantly correlated with the presence of local or distant metastasis in invasive breast carcinomas (11). These reports have been based on a study performed by Folkman et al (6), which suggested that tumor growth is angiogenesis-dependent, with a large amount of experimental evidence has supported this finding. Quantitative histological studies have suggested that angiogenesis correlates with the prognosis of human tumors, including breast carcinomas, but this point has been disputed.
In the current study, the microvessel counts showed no significant association with either the tumor size or lymph node status. However, the microvessel density revealed statistical significance with the nuclear grade, and tended to be higher with increasing histological grade. No association was found between the microvessel counts and DCIS component. With respect to the estrogen and progesterone receptor and c-erbB-2 stati, no significant associations were found in the quantification analysis of the microvessel counts.
Much experimental evidence has suggested that angiogenesis could facilitate the expansion of a primary tumor and increase its proliferative rate. Microvessel proliferation could increase the vascular area, which is the target of the invasive cancer cells, thereby facilitating the metastatic process (12). This step may be augmented by the leaky and immature nature of newly formed blood vessels, making the vascular invasion process easier. The above mechanism is supported by the fact cancer cells hardly invade blood vessels in the absence of neovascularization. In addition, according to the Uzzan et al (13), who performed a systematic review of the literature and meta-analysis, a high microvessel density significantly predicted poor survivals, both relapse free and overall. They concluded that although variations between studies could result from the patient selection criteria, the techniques used to stain and count the microvessels and the selection of cutoff values, the microvessel density was a significant prognostic factor in women with breast cancer.
However, the results of our investigation found no association between an increased microvessel density in invasive breast carcinomas and the clinically well-known prognostic factors of tumor size and lymph node status. Also, with respect to the DCIS component, CD34 positive microvessels were also noted around the component, and clearly considered incapable of metastasizing or showing tumor aggressiveness. Thus, even though angiogenesis may be necessary for metastasis or tumor aggressiveness, it does not appear to be sufficient as an independent factor of breast carcinoma invasiveness; therefore, other factors must be considered. With regard to the histopathological evaluation, the microvessel quantification showed significant association with the nuclear and histological grades, which are features of the morphological aggressiveness of an invasive breast carcinoma.
It has been reported that angiogenesis in malignant tumors may be induced by tumor cells via secretion of various types of angiogenic factors and inflammatory cells, including mast cells, via secretion of their angiogenic factors (14). Also, the expression of cyclooxygenease-2 in human breast carcinomas may play a role in tumor angiogenesis (15). It has also been reported that mast cells are conspicuously associated with angiogenesis, as they are found in chronic inflammatory diseases, for example, rheumatoid arthritis and psoriasis, and in tumors, such as hemangiomas, carcinomas, lymphomas and multiple myelomas (14,16). Histamine and tryptase, two other mast cell-derived factors, also stimulate angiogenesis. Mast cells have been reported as producing a variety of multifunctional cytokines and growth factors, such as IL-6 and IL-8, TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), TGF-β, FGF-2 and VEGF-A (17~20). Also, mast cells may contain heparin, in secretory granules, which has been shown to stimulate endothelial cell proliferation and migration in vitro (21), but have variable effects on angiogenesis in vivo, thus stimulating, inhibiting, or having no effect (22,23).
In the comparative analyses between the mast cell counts and microvessel density performed in this study, the number of mast cells was significantly increased with higher microvessel densities. We think that mast cells contribute to angiogenesis in invasive breast carcinoma, and also found mast cell counts to be significantly associated with the estrogen/progesterone receptor stati, with negatively correlations. In the study of Dabiri et al (24), the hormone receptor status (both estrogen receptor and progesterone receptor) was found to be correlated with a good outcome, while the overexpression of c-erbB-2 was associated with a poor outcome, and the presence of mast cells in the stroma were also correlated with a good prognosis (24). Considering the mast cell counts showed an inverse relationship with the hormomes' receptor stati in our study, the presence of increased mast cells could be suggested to have a relationship with a rather poor outcome.
In this study, no significant association was found between the mast cell counts and other parameters, including tumor size, lymph nodes status, nuclear and histological grades and DCIS component, even when the number of mast cells showed an increasing trend as the nuclear and histological grades became higher.
Macrophages had no correlation with the parameters analyzed in this study, with the exception of the progesterone receptor status, but a significantly negative correlation was shown with the protesterone (progesterone?) receptor status. As noted above, the macrophage increment could be presumed to be related with a poor outcome.
Macrophages are recruited to tumors through the local expression of potent chemoattractants, such as colony stimulating factor 1 (CSF-1) and macrophage chemoattractant protein 1, where their normal trophic functions are subverted to promote tumor progression and metastasis. In an experimental study on mice, the absence of macrophages did not change the incidence or growth of the primary tumor, but decreased the rate of progression and inhibited metastasis (25). We think that the abundance of tumor associated macrophages is correlated with poor prognosis.
In addition, mast cells and macrophages were positively and significantly correlated with each other. Therefore, we think that malignant tumor cells of invasive breast carcinoma recruit inflammatory cells, such as mast cells and macrophages, which contribute to angiogenesis.
This study suggests that increases numbers of mast cells and macrophages may be recruited and activated by malignant tumor cells, which contribute to angiogenesis in invasive breast carcinomas. Mast cells, however, were correlated with the nuclear grade, and also had a significant correlation with the estrogen and progesterone receptor stati. Macrophages showed a negative correlation with progesterone receptor status. Angiogenesis in invasive breast carcinomas had no significant association with the accepted prognostic parameters, such as tumor size, lymph node status, histological grade, the presence of the DCIS component and the estrogen/progesterone receptor and c-erbB-2 stati, but had a significant positive correlation with the nuclear grade. We think our angiogenesis evaluation using the microvessel density does not provide an independent clinicopathological factor for invasive breast carcinomas.
ACKNOWLEDGMENTS
The research was supported by the Chung-Ang University research grants in 2004 to E.S. Park
References
1. Polverini PJ. How the extracellular matrix and macrophages contribute to angiogenesis-dependent diseases. Eur J Cancer. 1996; 32A:2430–2437. PMID: 9059331.

2. Kessler DA, Langer RS, Pless NA, Folkman J. Mast cells ant tumor angiogenesis. Int J Cancer. 1976; 18:703–709. PMID: 62725.
3. Starkey JR, Crowle PK, Taubenberger S. Mast cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer. 1988; 42:48–52. PMID: 2455691.
4. Morra B, Ferrero V, Bussi M, Pacchioni D, Cerrato M, Bussolati G. Peri and intratumoral macrophage infiltration in laryngeal carcinoma. An immunohistochemical study. Acta Otolaryngol. 1991; 111:444–448. PMID: 2068934.
5. Bo L, Mork SJ, Nyland H. An immunohistochemical study of mononuclear cells in meningiomas. Neuropathol Appl Neurobiol. 1992; 18:548–558. PMID: 1488087.

6. Folkman J. Clinical applications of research on angiogenesis. N Engl J Med. 1995; 333:1757–1763. PMID: 7491141.

7. Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993; 73:161–195. PMID: 8419965.

8. Hildenbrand R, Dilger I, Horlin A, Stutte HJ. Urokinase and macrophages in tumour angiogenesis. Br J Cancer. 1995; 72:818–823. PMID: 7547226.

9. Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996; 56:4625–4629. PMID: 8840975.
10. Bigler SA, Deering RE, Brawer MK. Comparison of microscopic vascularity in benign and malignant prostate tissue. Hum Pathol. 1993; 24:220–226. PMID: 8432518.

11. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med. 1991; 324:1–8. PMID: 1701519.
12. Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 1974; 34:997–1004. PMID: 4841969.
13. Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004; 64:2941–2955. PMID: 15126324.
14. Cozzolino F, Torcia M, Aldinucci D, Rubartelli A, Miliani A, Shaw AR, et al. Production of interleukin-1 by bone marrow myeloma cells. Blood. 1989; 74:380–387. PMID: 2665838.

15. Kang HJ, Gong G, Jang SJ, Jung PJ, Park CK. Expression of cyclooxygenase-2 in human breast carcinoma: Relevance to tumor angiogenesis and expression of estrogen receptor. Cancer Res Treat. 2001; 33:286–295.

16. Meininger CJ, Zetter BR. Mast cells and angiogenesis. Semin Cancer Biol. 1992; 3:73–79. PMID: 1378312.
17. Qu Z, Liebler JM, Powers MR, Galey T, Ahmadi P, Huang XN, et al. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. 1995; 147:564–573. PMID: 7545872.
18. Motro B, Itin A, Sachs L, Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci USA. 1990; 87:3092–3096. PMID: 1691500.

19. Bussolino F, Ziche M, Wang JM, Alessi D, Morbidelli L, Cremona O, et al. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J Clin Invest. 1991; 87:986–995. PMID: 1705569.

20. Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986; 83:4167–4171. PMID: 2424019.

21. Raju KS, Alessandri G, Gullino PM. Characterization of a chemoattractant for endothelium induced by angiogenesis effectors. Cancer Res. 1984; 44:1579–1584. PMID: 6200213.
22. Norrby K. Heparin and angiogenesis: a low-molecular-weight fraction inhibits and a high-molecular-weight fraction stimulates angiogenesis systemically. Haemostasis. 1993; 23(Suppl. 1):141–149. PMID: 7684350.

23. Wilks JW, Scott PS, Vrba LK, Cocuzza JM. Inhibition of angiogenesis with combination treatments of angiostatic steroids and suramin. Int J Radiat Biol. 1991; 60:73–77. PMID: 1713944.

24. Dabiri S, Huntsman D, Makretsov N, Cheang M, Gilks B, Bajdik C, et al. The presence of stromal mast cells identifies a subset of invasive breast cancers with a favorable prognosis. Mod Pathol. 2004; 17:690–695. PMID: 15044916.

25. Lin EY, Pollard JW. Macrophages: modulators of breast cancer progression. Novartis Found Symp. 2004; 256:158–168. PMID: 15027489.

Fig. 1
Mast cells are highlighted with toluidine blue stain in the peritumoral area (Toluidine blue, ×400).

Fig. 2
CD68 positive macrophages are infiltrating into breast carcinoma cells and between tumor cell nests (×400).

Fig. 4
Mast cell counts in comparison with microvessel counts in 45 invasive breast carcinomas (Pearson's (r) coefficient=0.362, p-value=0.015).

Fig. 5
Macrophage counts in comparison with microvessel counts in 45 invasive breast carcinomas (Pearson's (r) coefficient=0.155, p-value=0.310).

Fig. 6
Macrophage counts in comparison with mast cell counts in 45 invasive breast carcinomas (Pearson's (r) coefficient=0.298, p-value=0.047).

Table 1
Correlation of mast cell, macrophage and microvessel counts to tumor size and lymph node metastasis




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download