1. Simon P. Simon P, editor. Basic Principles of Cancer Cell Culture. Methods in Molecular Medicine, Cancer Cell Culture: Methods and Protocols. 2004. 88. Totowa: Humana Press;p. 3–15.
2. Park JG, Frucht H, LaRocca RV, Bliss DP Jr, Kurita Y, Chen TR, et al. Characteristics of cell lines established from human gastric carcinoma. Cancer Res. 1990; 50:2773–2780. PMID:
2158397.
3. Park JG, Lee JH, Kang MS, Park KJ, Jeon YM, Lee HJ, et al. Characterization of cell lines established from human hepatocellular carcinoma. Int J Cancer. 1995; 62:276–282. PMID:
7543080.

4. Park JG, Oie HK, Sugarbaker PH, Henslee JG, Chen TR, Johnson BE, et al. Characteristics of cell lines established from human colorectal carcinoma. Cancer Res. 1987; 47:6710–6718. PMID:
3479249.
5. Park JG, Yang HK, Kim WH, Chung JK, Kang MS, Lee JH, et al. Establishment and characterization of human gastric carcinoma cell lines. Int J Cancer. 1997; 70:443–449. PMID:
9033653.

6. Oh JH, Ku JL, Yoon KA, Kwon HJ, Kim WH, Park HS, et al. Establishment and characterization of 12 human colorectal-carcinoma cell lines. Int J Cancer. 1999; 81:902–910. PMID:
10362137.

7. Lee JH, Ku JL, Park YJ, Lee KU, Kim WH, Park JG. Establishment and characterization of four human hepatocellular carcinoma cell lines containing hepatitis B virus DNA. World J Gastroenterol. 1999; 5:289–295. PMID:
11819450.

8. Ku JL, Kim WH, Park HS, Kang SB, Park JG. Establishment and characterization of 12 uterine cervical-carcinoma cell lines: common sequence variation in the E7 gene of HPV-16-positive cell lines. Int J Cancer. 1997; 72:313–320. PMID:
9219839.
9. Lee WK, Kim SM, Sim YS, Cho SG, Park SH, Kim CW, et al. B-lymphoblastoid cell lines from cancer patients. In Vitro Cell Dev Biol Anim. 1998; 34:97–100. PMID:
9542645.

10. Yuan Y, Kim WH, Han HS, Lee JH, Park HS, Chung JK, et al. Establishment and characterization of human ovarian carcinoma cell lines. Gynecol Oncol. 1997; 66:378–387. PMID:
9299249.

11. Yuan Y, Kim WH, Han HS, Lee JH, Park HS, Chung JK, et al. Establishment and characterization of cell lines derived from uterine malignant mixed Mullerian tumor. Gynecol Oncol. 1997; 66:464–474. PMID:
9299262.
12. Ku JL, Kim WH, Lee JH, Park HS, Kim KH, et al. Establishment and characterization of human laryngeal squamous cell carcinoma cell lines. Laryngoscope. 1999; 109:976–982. PMID:
10369293.

13. Shin KH, Ku JL, Kim WH, Lee SE, Lee C, Kim SW, et al. Establishment and characterization of seven human renal cell carcinoma cell lines. BJU Int. 2000; 85:130–138. PMID:
10619961.

14. Shin KH, Choe G, Park YJ, Jang JH, Jung HW, Park JG. Establishment and characterization of nine human brain tumor cell lines. In Vitro Cell Dev Biol Anim. 2001; 37:625–628. PMID:
11776963.
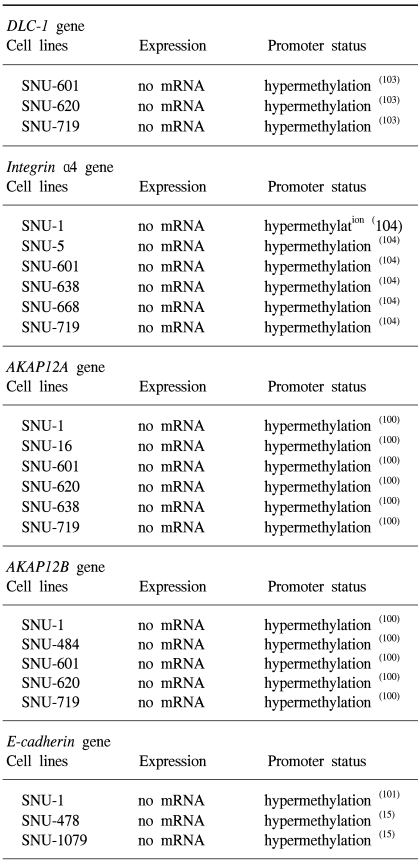
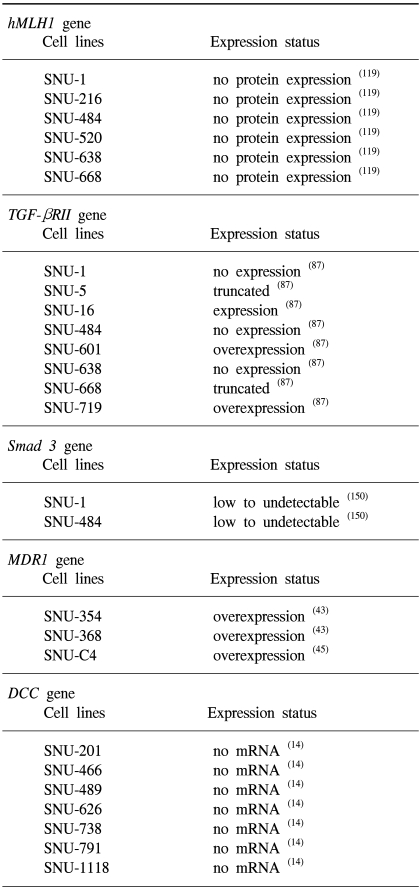
15. Ku JL, Yoon KA, Kim IJ, Kim WH, Jang JY, Suh KS, et al. Establishment and characterisation of six human biliary tract cancer cell lines. Br J Cancer. 2002; 87:187–193. PMID:
12107841.

16. Ku JL, Yoon KA, Kim WH, Jang Y, Suh KS, Kim SW, et al. Establishment and characterization of four human pancreatic carcinoma cell lines. Genetic alterations in the TGFBR2 gene but not in the MADH4 gene. Cell Tissue Res. 2002; 308:205–214. PMID:
12037578.
17. Kang HC, Kim IJ, Park JH, Shin Y, Ku JL, Jung MS, et al. Identification of genes with differential expression in acquired drug-resistant gastric cancer cells using high-density oligonucleotide microarrays. Clin Cancer Res. 2004; 10:272–284. PMID:
14734480.

18. Kang MS, Kim HS, Han JA, Park SC, Kim WB, Park JG. Characteristics of human gastric carcinoma cell lines with induced multidrug resistance. Anticancer Res. 1997; 17:3531–3536. PMID:
9413198.
19. Yoo BC, Jeon E, Hong SH, Shin YK, Chang HJ, Park JG. Metabotropic glutamate receptor 4-mediated 5-Fluorouracil resistance in a human colon cancer cell line. Clin Cancer Res. 2004; 10:4176–4184. PMID:
15217955.

20. Yoo BC, Ku JL, Hong SH, Shin YK, Park SY, Kim HK, et al. Decreased pyruvate kinase M2 activity linked to cisplatin resistance in human gastric carcinoma cell lines. Int J Cancer. 2004; 108:532–539. PMID:
14696117.

21. Yoon KA, Ku JL, Yang JO, Park JG. Telomerase activity, expression of Bcl-2 and cell cycle regulation in doxorubicin resistant gastric carcinoma cell lines. Int J Mol Med. 2003; 11:343–348. PMID:
12579337.

22. Park JG. SNU cell lines and their application for cancer research. Gan To Kagaku Ryoho. 2002; 29(Suppl 1):89–98. PMID:
11890121.
23. Park JG, Ku JL, Park SY. Simon P, editor. Isolation and culture of renal cancer cell lines. Methods in Molecular Medicine, Cancer Cell Culture: Methods and Protocols. 2004. 88. Totowa: Humana Press;p. 111–119.

24. Park JG, Ku JL, Park SY. Simon P, editor. Isolation and culture of colon cancer cell lines. Methods in Molecular Medicine, Cancer Cell Culture: Methods and Protocols. 2004. 88. Totowa: Humana Press;p. 79–92.

25. Park JG, Ku JL, Kim HS, Park SY, Rutten MJ. Pfragner R FR, editor. Culture of normal and malignant gastric epithelium. Culture of human tumor cells. 2004. Hoboken: John Wiley & Sons;p. 23–66.

26. Carmichael J, Park JG, Degraff WG, Gamson J, Gazdar AF, Mitchell JB. Radiation sensitivity and study of glutathione and related enzymes in human colorectal cancer cell lines. Eur J Cancer Clin Oncol. 1988; 24:1219–1224. PMID:
2901354.

27. Cheng K, Chen Y, Zimniak P, Raufman JP, Xiao Y, Frucht H. Functional interaction of lithocholic acid conjugates with M3 muscarinic receptors on a human colon cancer cell line. Biochim Biophys Acta. 2002; 1588:48–55. PMID:
12379313.

28. Frucht H, Gazdar AF, Park JA, Oie H, Jensen RT. Characterization of functional receptors for gastrointestinal hormones on human colon cancer cells. Cancer Res. 1992; 52:1114–1122. PMID:
1310640.
29. Grem JL, Allegra CJ. Toxicity of levamisole and 5-fluorouracil in human colon carcinoma cells. J Natl Cancer Inst. 1989; 81:1413–1417. PMID:
2778828.

30. Grem JL, Voeller DM, Geoffroy F, Horak E, Johnston PG, Allegra CJ. Determinants of trimetrexate lethality in human colon cancer cells. Br J Cancer. 1994; 70:1075–1084. PMID:
7981057.

31. Karnes WE Jr, Walsh JH, Wu SV, Kim RS, Martin MG, Wong HC, et al. Autonomous proliferation of colon cancer cells that coexpress transforming growth factor alpha and its receptor. Variable effects of receptor-blocking antibody. Gastroenterology. 1992; 102:474–485. PMID:
1732118.
32. Kim HS, Lee BL, Bae SI, Kim YI, Park JG, Kleinman HK, et al. Differentiation of a colon cancer cell line on a reconstituted basement membrane in vitro. Int J Exp Pathol. 1998; 79:443–451. PMID:
10319025.

33. Kim IJ, Kang HC, Park JH, Shin Y, Ku JL, Lim SB, et al. Development and applications of a beta-catenin oligonucleotide microarray: beta-catenin mutations are dominantly found in the proximal colon cancers with microsatellite instability. Clin Cancer Res. 2003; 9:2920–2925. PMID:
12912937.
34. Kim IJ, Ku JL, Kang HC, Park JH, Yoon KA, Shin Y, et al. Mutational analysis of OGG1, MYH, MTH1 in FAP, HNPCC and sporadic colorectal cancer patients: R154H OGG1 polymorphism is associated with sporadic colorectal cancer patients. Hum Genet. 2004; 115:498–503. PMID:
15449173.

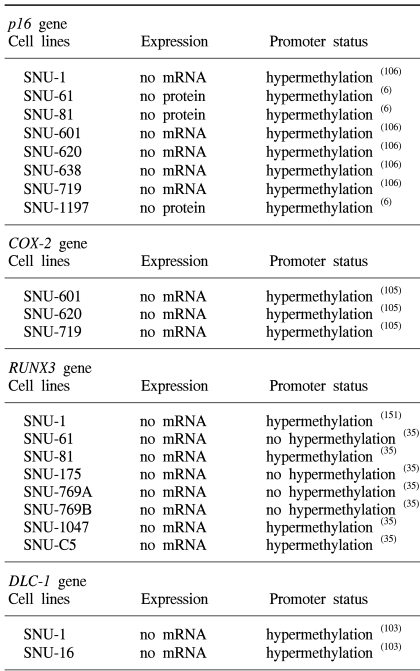
35. Ku JL, Kang SB, Shin YK, Kang HC, Hong SH, Kim IJ, et al. Promoter hypermethylation downregulates RUNX3 gene expression in colorectal cancer cell lines. Oncogene. 2004; 23:6736–6742. PMID:
15273736.

36. Ku JL, Yoon KA, Kim DY, Park JG. Mutations in hMSH6 alone are not sufficient to cause the microsatellite instability in colorectal cancer cell lines. Eur J Cancer. 1999; 35:1724–1729. PMID:
10674020.

37. La Rocca RV, Park JG, Danesi R, Del Tacca M, Steinberg SM, Gazdar AF. Pattern of growth factor, proto-oncogene and carcinoembryonic antigen gene expression in human colorectal carcinoma cell lines. Oncology. 1992; 49:209–214. PMID:
1495747.

38. Mans DR, Grivicich I, Peters GJ, Schwartsmann G. Sequence-dependent growth inhibition and DNA damage formation by the irinotecan-5-fluorouracil combination in human colon carcinoma cell lines. Eur J Cancer. 1999; 35:1851–1861. PMID:
10674003.

39. Min JJ, Chung JK, Lee YJ, Shin JH, Yeo JS, Jeong JM, et al. In vitro and in vivo characteristics of a human colon cancer cell line, SNU-C5N, expressing sodium-iodide symporter. Nucl Med Biol. 2002; 29:537–545. PMID:
12088723.

40. Park JG, Collins JM, Gazdar AF, Allegra CJ, Steinberg SM, Greene RF, et al. Enhancement of fluorinated pyrimidine-induced cytotoxicity by leucovorin in human colorectal carcinoma cell lines. J Natl Cancer Inst. 1988; 80:1560–1564. PMID:
2973527.

41. Park JG, Gazdar AF. Biology of colorectal and gastric cancer cell lines. J Cell Biochem Suppl. 1996; 24:131–141. PMID:
8806095.

42. Park JG, Kramer BS, Steinberg SM, Carmichael J, Collins JM, Minna JD, et al. Chemosensitivity testing of human colorectal carcinoma cell lines using a tetrazolium-based colorimetric assay. Cancer Res. 1987; 47:5875–5879. PMID:
3664487.
43. Park JG, Lee SK, Hong IG, Kim HS, Lim KH, Choe KJ, et al. MDR1 gene expression: its effect on drug resistance to doxorubicin in human hepatocellular carcinoma cell lines. J Natl Cancer Inst. 1994; 86:700–705. PMID:
7908989.

44. Park JH, Kim IJ, Kang HC, Shin Y, Park HW, Jang SG, et al. Oligonucleotide microarray-based mutation detection of the K-ras gene in colorectal cancers with use of competitive DNA hybridization. Clin Chem. 2004; 50:1688–1691. PMID:
15331511.

45. Park JG, Kramer BS, Lai SL, Goldstein LJ, Gazdar AF. Chemosensitivity patterns and expression of human multidrug resistance-associated MDR1 gene by human gastric and colorectal carcinoma cell lines. J Natl Cancer Inst. 1990; 82:193–198. PMID:
1967320.

46. Grem JL, Allegra CJ. Sequence-dependent interaction of 5-fluorouracil and arabinosyl-5-azacytosine or 1-beta-D-arabinofuranosylcytosine. Biochem Pharmacol. 1991; 42:409–418. PMID:
1713459.
47. Lim KH, Kim HS, Yang YM, Lee SD, Kim WB, Yang J, et al. Cellular uptake and antitumor activity of the new anthracycline analog DA-125 in human cancer cell lines. Cancer Chemother Pharmacol. 1997; 40:23–30. PMID:
9137525.

48. Shibata J, Aiba K, Shibata H, Minowa S, Horikoshi N. [Detection of thymidylate synthase mRNA in 5-fluorouracil resistant human colon adenocarcinoma cells]. Gan To Kagaku Ryoho. 1994; 21:1613–1618. PMID:
8060136.
49. Shibata J, Aiba K, Shibata H, Minowa S, Horikoshi N. Detection and quantitation of thymidylate synthase mRNA in human colon adenocarcinoma cell line resistant to 5-fluorouracil by competitive PCR. Anticancer Res. 1998; 18:1457–1463. PMID:
9673356.
50. Yee LK, Allegra CJ, Trepel JB, Grem JL. Metabolism and RNA incorporation of cyclopentenyl cytosine in human colorectal cancer cells. Biochem Pharmacol. 1992; 43:1587–1599. PMID:
1567480.

51. Nam KS, Jo YS, Kim YH, Hyun JW, Kim HW. Cytotoxic activities of acetoxyscirpenediol and ergosterol peroxide from Paecilomyces tenuipes. Life Sci. 2001; 69:229–237. PMID:
11441913.

52. Ryu EK, Choe YS, Byun SS, Lee KH, Chi DY, Choi Y, et al. Synthesis of radioiodine labeled dibenzyl disulfide for evaluation of tumor cell uptake. Bioorg Med Chem. 2004; 12:859–864. PMID:
14980597.

53. Seo BR, Yoo CB, Park HJ, Choi JW, Seo K, Choi SK, et al. Saucernetin-8 Isolated from Saururus chinensis Induced the Differentiation of Human Acute Promyelocytic Leukemia HL-60 Cells. Biol Pharm Bull. 2004; 27:1594–1598. PMID:
15467202.

54. Tsai CM, Gazdar AF, Perng RP, Kramer BS. Schedule-dependent in vitro combination effects of methotrexate and 5-fluorouracil in human tumor cell lines. Int J Cancer. 1991; 47:401–407. PMID:
1847123.
55. Shin JH, Shin YK, Ku JL, Jeong SY, Hong SH, Park SY, et al. Mutations of the Birt-Hogg-Dube (BHD) gene in sporadic colorectal carcinomas and colorectal carcinoma cell lines with microsatellite instability. J Med Genet. 2003; 40:364–367. PMID:
12746401.

56. Helman LJ, Gazdar AF, Park JG, Cohen PS, Cotelingam JD, Israel MA. Chromogranin A expression in normal and malignant human tissues. J Clin Invest. 1988; 82:686–690. PMID:
3403722.

57. Nagayama S, Iiizumi M, Katagiri T, Toguchida J, Nakamura Y. Identification of PDZK4, a novel human gene with PDZ domains, that is upregulated in synovial sarcomas. Oncogene. 2004; 23:5551–5557. PMID:
15077175.

58. Shin Y, Kim IJ, Kang HC, Park JH, Park HR, Park HW, et al. The E-cadherin -347G→GA promoter polymorphism and its effect on transcriptional regulation. Carcinogenesis. 2004; 25:895–899. PMID:
14729585.

59. Shin Y, Kim IJ, Kang HC, Park JH, Park HW, Jang SG, et al. A functional polymorphism (-347 G→GA) in the E-cadherin gene is associated with colorectal cancer. Carcinogenesis. 2004; 25:2173–2176. PMID:
15231691.

60. Park JG, Choe GY, Helman LJ, Gazdar AF, Yang HK, Kim JP, et al. Chromogranin-A expression in gastric and colon cancer tissues. Int J Cancer. 1992; 51:189–194. PMID:
1349007.

61. Silverman AL, Park JG, Hamilton SR, Gazdar AF, Luk GD, Baylin SB. Abnormal methylation of the calcitonin gene in human colonic neoplasms. Cancer Res. 1989; 49:3468–3473. PMID:
2731168.
62. Rand A, Glenn KS, Alvares CP, White MB, Thibodeau SM, Karnes WE Jr. p53 functional loss in a colon cancer cell line with two missense mutations (218leu and 248trp) on separate alleles. Cancer Lett. 1996; 98:183–191. PMID:
8556707.

63. Jang JH, Shin KH, Park YJ, Lee RJ, McKeehan WL, Park JG. Novel transcripts of fibroblast growth factor receptor 3 reveal aberrant splicing and activation of cryptic splice sequences in colorectal cancer. Cancer Res. 2000; 60:4049–4052. PMID:
10945607.
64. Ahn CM, Kim SK, Han JL. Synthesis of 6-aziridinylbenzimidazole derivatives and their in vitro antitumor activities. Arch Pharm Res. 1998; 21:599–609. PMID:
9875502.
65. Ahn CM, Tak JA, Choi SJ. Synthesis and in vitro antitumor activity of 2-alkyl, 2-aryl, and 2-piperazinyl benzimidazole-4,7-dione derivatives. Arch Pharm Res. 2000; 23:288–301. PMID:
10976573.
66. Buell JF, Reed E, Lee KB, Parker RJ, Venzon DJ, Amikura K, et al. Synergistic effect and possible mechanisms of tumor necrosis factor and cisplatin cytotoxicity under moderate hyperthermia against gastric cancer cells. Ann Surg Oncol. 1997; 4:141–148. PMID:
9084851.

67. Iseki H, Ko TC, Xue XY, Seapan A, Hellmich MR, Townsend CM Jr. Cyclin-dependent kinase inhibitors block proliferation of human gastric cancer cells. Surgery. 1997; 122:187–194. discussion 194-85. PMID:
9288122.

68. Kim SG, Kim SN, Jong HS, Kim NK, Hong SH, Kim SJ, et al. Caspase-mediated Cdk2 activation is a critical step to execute transforming growth factor-beta1-induced apoptosis in human gastric cancer cells. Oncogene. 2001; 20:1254–1265. PMID:
11313870.
69. Lee KT, Sohn IC, Park HJ, Kim DW, Jung GO, Park KY. Essential moiety for antimutagenic and cytotoxic activity of hederagenin monodesmosides and bisdesmosides isolated from the stem bark of Kalopanax pictus. Planta Med. 2000; 66:329–332. PMID:
10865448.

70. Okabe S, Ochiai Y, Aida M, Park K, Kim SJ, Nomura T, et al. Mechanistic aspects of green tea as a cancer preventive: effect of components on human stomach cancer cell lines. Jpn J Cancer Res. 1999; 90:733–739. PMID:
10470285.

71. Park IC, Park MJ, Choe TB, Jang JJ, Hong SI, Lee SH. TNF-alpha induces apoptosis mediated by AEBSF-sensitive serine protease (s) that may involve upstream caspase-3/CPP32 protease activation in a human gastric cancer cell line. Int J Oncol. 2000; 16:1243–1248. PMID:
10812002.
72. Quasney ME, Carter LC, Oxford C, Watkins SM, Gershwin ME, German JB. Inhibition of proliferation and induction of apoptosis in SNU-1 human gastric cancer cells by the plant sulfolipid, sulfoquinovosyldiacylglycerol. J Nutr Biochem. 2001; 12:310–315. PMID:
11382550.

73. Yoo YD, Park JK, Choi JY, Lee KH, Kang YK, Kim CS, et al. CDK4 down-regulation induced by paclitaxel is associated with G1 arrest in gastric cancer cells. Clin Cancer Res. 1998; 4:3063–3068. PMID:
9865921.
74. Lee KS, Kim HK, Moon HS, Hong YS, Kang JH, Kim DJ, et al. Effects of buthionine sulfoximine treatment on cellular glutathione levels and cytotoxicities of cisplatin, carboplatin and radiation in human stomach and ovarian cancer cell lines. Korean J Intern Med. 1992; 7:111–117. PMID:
1306072.
75. Choe G, Kim WH, Park JG, Kim YI. Effect of suramin on differentiation of human stomach cancer cell lines. J Korean Med Sci. 1997; 12:433–442. PMID:
9364302.

76. Chung Y, Shin YK, Zhan CG, Lee S, Cho H. Synthesis and evaluation of antitumor activity of 2- and 6-[(1,3-benzothiazol-2-yl) aminomethyl]-5, 8-dimethoxy-1, 4-naphthoquinone derivatives. Arch Pharm Res. 2004; 27:893–900. PMID:
15473656.
77. Jang TJ, Kang HJ, Kim JR, Yang CH. Non-steroidal antiinflammatory drug activated gene (NAG-1) expression is closely related to death receptor-4 and -5 induction, which may explain sulindac sulfide induced gastric cancer cell apoptosis. Carcinogenesis. 2004; 25:1853–1858. PMID:
15180942.

78. Kim DK, Kim G, Gam J, Cho YB, Kim HT, Tai JH, et al. Synthesis and antitumor activity of a series of [2-substituted-4, 5-bis (aminomethyl)-1, 3-dioxolane] platinum (II) complexes. J Med Chem. 1994; 37:1471–1485. PMID:
8182706.
79. Kim DK, Kim HT, Tai JH, Cho YB, Kim TS, Kim KH, et al. Pharmacokinetics and antitumor activity of a new platinum compound, cis-malonato [(4R,5R)-4, 5-bis (aminomethyl)-2-isopropyl-1, 3-dioxolane] platinum (II), as determined by ex vivo pharmacodynamics. Cancer Chemother Pharmacol. 1995; 37:1–6. PMID:
7497577.
80. Park HJ, Lee HJ, Lee EJ, Hwang HJ, Shin SH, Suh ME, et al. Cytotoxicity and DNA topoisomerase inhibitory activity of benz [f] indole-4, 9-dione analogs. Biosci Biotechnol Biochem. 2003; 67:1944–1949. PMID:
14519980.
81. Yang YM, Hyun JW, Sung MS, Chung HS, Kim BK, Paik WH, et al. The cytotoxicity of psoralidin from Psoralea corylifolia. Planta Med. 1996; 62:353–354. PMID:
8792669.
82. Chung YM, Park S, Park JK, Kim Y, Kang Y, Yoo YD. Establishment and characterization of 5-fluorouracil-resistant gastric cancer cells. Cancer Lett. 2000; 159:95–101. PMID:
10974411.

83. Chang J, Park K, Bang YJ, Kim WS, Kim D, Kim SJ. Expression of transforming growth factor beta type II receptor reduces tumorigenicity in human gastric cancer cells. Cancer Res. 1997; 57:2856–2859. PMID:
9230189.
84. Kang SH, Bang YJ, Im YH, Yang HK, Lee DA, Lee HY, et al. Transcriptional repression of the transforming growth factor-beta type I receptor gene by DNA methylation results in the development of TGF-beta resistance in human gastric cancer. Oncogene. 1999; 18:7280–7286. PMID:
10602482.
85. Kang SH, Bang YJ, Jong HS, Seo JY, Kim NK, Kim SJ. Rapid induction of p21WAF1 but delayed down-regulation of Cdc 25A in the TGF-beta-induced cell cycle arrest of gastric carcinoma cells. Br J Cancer. 1999; 80:1144–1149. PMID:
10376964.
86. Myeroff LL, Parsons R, Kim SJ, Hedrick L, Cho KR, Orth K, et al. A transforming growth factor beta receptor type II gene mutation common in colon and gastric but rare in endometrial cancers with microsatellite instability. Cancer Res. 1995; 55:5545–5547. PMID:
7585631.
87. Park K, Kim SJ, Bang YJ, Park JG, Kim NK, Roberts AB, et al. Genetic changes in the transforming growth factor beta (TGF-beta) type II receptor gene in human gastric cancer cells: correlation with sensitivity to growth inhibition by TGF-beta. Proc Natl Acad Sci U S A. 1994; 91:8772–8776. PMID:
8090721.

88. Yang HK, Kang SH, Kim YS, Won K, Bang YJ, Kim SJ. Truncation of the TGF-beta type II receptor gene results in insensitivity to TGF-beta in human gastric cancer cells. Oncogene. 1999; 18:2213–2219. PMID:
10327067.
89. Kim JD, Kim JM, Pyo JO, Kim SY, Kim BS, Yu R, et al. Capsaicin can alter the expression of tumor forming-related genes which might be followed by induction of apoptosis of a Korean stomach cancer cell line, SNU-1. Cancer Lett. 1997; 120:235–241. PMID:
9461043.

90. Ju HR, Jung U, Sonn CH, Yoon SR, Jeon JH, Yang Y, et al. Aberrant signaling of TGF-beta1 by the mutant Smad4 in gastric cancer cells. Cancer Lett. 2003; 196:197–206. PMID:
12860278.
91. Kim SG, Jong HS, Kim TY, Lee JW, Kim NK, Hong SH, et al. Transforming growth factor-beta 1 induces apoptosis through Fas ligand-independent activation of the Fas death pathway in human gastric SNU-620 carcinoma cells. Mol Biol Cell. 2004; 15:420–434. PMID:
14595120.
92. Bar-Am I, Mor O, Yeger H, Shiloh Y, Avivi L. Detection of amplified DNA sequences in human tumor cell lines by fluorescence in situ hybridization. Genes Chromosomes Cancer. 1992; 4:314–320. PMID:
1377938.

93. Hara T, Ooi A, Kobayashi M, Mai M, Yanagihara K, Nakanishi I. Amplification of c-myc, K-sam, and c-met in gastric cancers: detection by fluorescence in situ hybridization. Lab Invest. 1998; 78:1143–1153. PMID:
9759658.
94. Mor O, Messinger Y, Rotman G, Bar-Am I, Ravia Y, Eddy RL, et al. Novel DNA sequences at chromosome 10q26 are amplified in human gastric carcinoma cell lines: molecular cloning by competitive DNA reassociation. Nucleic Acids Res. 1991; 19:117–123. PMID:
2011492.

95. Mor O, Ranzani GN, Ravia Y, Rotman G, Gutman M, Manor A, et al. DNA amplification in human gastric carcinomas. Cancer Genet Cytogenet. 1993; 65:111–114. PMID:
8453595.

96. Park IC, Park MJ, Lee SH, Choe TB, Jang JJ, Hong SI. Increased susceptibility of the c-Myc overexpressing cell line, SNU-16, to TNF-alpha. Cancer Lett. 1998; 125:17–23. PMID:
9566690.

97. Bae CD, Juhnn YS, Park JB. Post-transcriptional control of c-erb B-2 overexpression in stomach cancer cells. Exp Mol Med. 2001; 33:15–19. PMID:
11322480.

98. Bae CD, Park SE, Seong YS, Kimm SW, Park JB. The mechanism of c-erbB-2 gene product increase in stomach cancer cell lines. J Korean Med Sci. 1993; 8:153–159. PMID:
8104420.

99. Jong HS, Park YI, Kim S, Sohn JH, Kang SH, Song SH, et al. Up-regulation of human telomerase catalytic subunit during gastric carcinogenesis. Cancer. 1999; 86:559–565. PMID:
10440682.

100. Choi MC, Jong HS, Kim TY, Song SH, Lee DS, Lee JW, et al. AKAP12/Gravin is inactivated by epigenetic mechanism in human gastric carcinoma and shows growth suppressor activity. Oncogene. 2004; 23:7095–7103. PMID:
15258566.

101. Grady WM, Willis J, Guilford PJ, Dunbier AK, Toro TT, Lynch H, et al. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000; 26:16–17. PMID:
10973239.

102. Kang SH, Choi HH, Kim SG, Jong HS, Kim NK, Kim SJ, et al. Transcriptional inactivation of the tissue inhibitor of metalloproteinase-3 gene by dna hypermethylation of the 5'-CpG island in human gastric cancer cell lines. Int J Cancer. 2000; 86:632–635. PMID:
10797283.

103. Kim TY, Jong HS, Song SH, Dimtchev A, Jeong SJ, Lee JW, et al. Transcriptional silencing of the DLC-1 tumor suppressor gene by epigenetic mechanism in gastric cancer cells. Oncogene. 2003; 22:3943–3951. PMID:
12813468.

104. Park J, Song SH, Kim TY, Choi MC, Jong HS, Lee JW, et al. Aberrant methylation of integrin alpha4 gene in human gastric cancer cells. Oncogene. 2004; 23:3474–3480. PMID:
14990990.
105. Song SH, Jong HS, Choi HH, Inoue H, Tanabe T, Kim NK, et al. Transcriptional silencing of cyclooxygenase-2 by hyper-methylation of the 5' CpG island in human gastric carcinoma cells. Cancer Res. 2001; 61:4628–4635. PMID:
11389100.
106. Song SH, Jong HS, Choi HH, Kang SH, Ryu MH, Kim NK, et al. Methylation of specific CpG sites in the promoter region could significantly down-regulate p16(INK4a) expression in gastric adenocarcinoma. Int J Cancer. 2000; 87:236–240. PMID:
10861481.

107. Kang HC, Kim IJ, Park JH, Shin Y, Park HW, Ku JL, et al. Promoter hypermethylation and silencing of CHFR mitotic stress checkpoint gene in human gastric cancers. Oncol Rep. 2004; 12:129–133. PMID:
15201973.

108. Chun YH, Kil JI, Suh YS, Kim SH, Kim H, Park SH. Characterization of chromosomal aberrations in human gastric carcinoma cell lines using chromosome painting. Cancer Genet Cytogenet. 2000; 119:18–25. PMID:
10812166.

109. Fukuda Y, Kurihara N, Imoto I, Yasui K, Yoshida M, Yanagihara K, et al. CD44 is a potential target of amplification within the 11p13 amplicon detected in gastric cancer cell lines. Genes Chromosomes Cancer. 2000; 29:315–324. PMID:
11066075.

110. Schneider BG, Rha SY, Chung HC, Bravo JC, Mera R, Torres JC, et al. Regions of allelic imbalance in the distal portion of chromosome 12q in gastric cancer. Mol Pathol. 2003; 56:141–149. PMID:
12782760.

111. Jang JH, Shin KH, Park JG. Mutations in fibroblast growth factor receptor 2 and fibroblast growth factor receptor 3 genes associated with human gastric and colorectal cancers. Cancer Res. 2001; 61:3541–3543. PMID:
11325814.
112. Shin EY, Ma EK, Kim CK, Kwak SJ, Kim EG. Src/ERK but not phospholipase D is involved in keratinocyte growth factor-stimulated secretion of matrix metalloprotease-9 and urokinase-type plasminogen activator in SNU-16 human stomach cancer cell. J Cancer Res Clin Oncol. 2002; 128:596–602. PMID:
12458339.

113. Hwang IR, Hsu PI, Peterson LE, Gutierrez O, Kim JG, Graham DY, et al. Interleukin-6 genetic polymorphisms are not related to Helicobacter pylori-associated gastroduodenal diseases. Helicobacter. 2003; 8:142–148. PMID:
12662382.

114. Jung HC, Kim JM, Song IS, Kim CY. Helicobacter pylori induces an array of pro-inflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin-8, -1 alpha/beta, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 and tumour necrosis factor-alpha. J Gastroenterol Hepatol. 1997; 12:473–480. PMID:
9257236.
115. Kim JM, Kim JS, Jung HC, Oh YK, Kim N, Song IS. Inhibition of Helicobacter pylori-induced nuclear factor-kappa B activation and interleukin-8 gene expression by ecabet sodium in gastric epithelial cells. Helicobacter. 2003; 8:542–553. PMID:
14536001.

116. Kim JM, Kim JS, Jung HC, Song IS, Kim CY. Upregulated cyclooxygenase-2 inhibits apoptosis of human gastric epithelial cells infected with Helicobacter pylori. Dig Dis Sci. 2000; 45:2436–2443. PMID:
11258572.
117. Kim JM, Kim JS, Jung HC, Song IS, Kim CY. Up-regulation of inducible nitric oxide synthase and nitric oxide in Helicobacter pylori-infected human gastric epithelial cells: possible role of interferon-gamma in polarized nitric oxide secretion. Helicobacter. 2002; 7:116–128. PMID:
11966872.

118. Shin KH, Han HJ, Park JG. Growth suppression mediated by transfection of wild-type hMLH1 in human cancer cells expressing endogenous truncated hMLH1 protein. Int J Oncol. 1998; 12:609–615. PMID:
9472100.

119. Shin KH, Yang YM, Park JG. Absence or decreased levels of the hMLH1 protein in human gastric carcinoma cell lines: implication of hMLH1 in alkylation tolerance. J Cancer Res Clin Oncol. 1998; 124:421–426. PMID:
9750018.

120. Park IC, Park MJ, Rhee CH, Lee JI, Choe TB, Jang JJ, et al. Protein kinase C activation by PMA rapidly induces apoptosis through caspase-3/CPP32 and serine protease(s) in a gastric cancer cell line. Int J Oncol. 2001; 18:1077–1083. PMID:
11295059.

121. Yang Z, Liu S, Chen X, Chen H, Huang M, Zheng J. Induction of apoptotic cell death and in vivo growth inhibition of human cancer cells by a saturated branched-chain fatty acid, 13-methyltetradecanoic acid. Cancer Res. 2000; 60:505–509. PMID:
10676625.
122. Nam SY, Jung GA, Hur GC, Chung HY, Kim WH, Seol DW, et al. Upregulation of FLIP(S) by Akt, a possible inhibition mechanism of TRAIL-induced apoptosis in human gastric cancers. Cancer Sci. 2003; 94:1066–1073. PMID:
14662022.

123. Lee TB, Min YD, Lim SC, Kim KJ, Jeon HJ, Choi SM, et al. Fas (Apo-1/CD95) and Fas ligand interaction between gastric cancer cells and immune cells. J Gastroenterol Hepatol. 2002; 17:32–38. PMID:
11895550.

124. Jeong JH, Park JS, Moon B, Kim MC, Kim JK, Lee S, et al. Orphan nuclear receptor Nur77 translocates to mitochondria in the early phase of apoptosis induced by synthetic chenodeoxycholic acid derivatives in human stomach cancer cell line SNU-1. Ann N Y Acad Sci. 2003; 1010:171–177. PMID:
15033715.

125. Park IK, Kim BJ, Goh YJ, Lyu MA, Park CG, Hwang ES, et al. Co-expression of urokinase-type plasminogen activator and its receptor in human gastric-cancer cell lines correlates with their invasiveness and tumorigenicity. Int J Cancer. 1997; 71:867–873. PMID:
9180158.

126. Rabenau KE, O'Toole JM, Bassi R, Kotanides H, Witte L, Ludwig DL, et al. DEGA/AMIGO-2, a leucine-rich repeat family member, differentially expressed in human gastric adenocarcinoma: effects on ploidy, chromosomal stability, cell adhesion/migration and tumorigenicity. Oncogene. 2004; 23:5056–5067. PMID:
15107827.

127. Shin BA, Yoo HG, Kim HS, Kim MH, Hwang YS, Chay KO, et al. P38 MAPK pathway is involved in the urokinase plasminogen activator expression in human gastric SNU-638 cells. Oncol Rep. 2003; 10:1467–1471. PMID:
12883725.

128. Lee DH, Yang Y, Lee SJ, Kim KY, Koo TH, Shin SM, et al. Macrophage inhibitory cytokine-1 induces the invasiveness of gastric cancer cells by up-regulating the urokinase-type plasminogen activator system. Cancer Res. 2003; 63:4648–4655. PMID:
12907645.
129. Cho YG, Nam SW, Kim TY, Kim YS, Kim CJ, Park JY, et al. Overexpression of S100A4 is closely related to the aggressiveness of gastric cancer. Apmis. 2003; 111:539–545. PMID:
12887505.

130. Zhang H, Wu J, Meng L, Shou CC. Expression of vascular endothelial growth factor and its receptors KDR and Flt-1 in gastric cancer cells. World J Gastroenterol. 2002; 8:994–998. PMID:
12439912.

131. Kim IJ, Park JH, Kang HC, Shin Y, Park HW, Park HR, et al. Mutational analysis of BRAF and K-ras in gastric cancers: absence of BRAF mutations in gastric cancers. Hum Genet. 2003; 114:118–120. PMID:
14513361.

132. Kim JH, Takahashi T, Chiba I, Park JG, Birrer MJ, Roh JK, et al. Occurrence of p53 gene abnormalities in gastric carcinoma tumors and cell lines. J Natl Cancer Inst. 1991; 83:938–943. PMID:
1676761.
133. Shin KH, Park JG. Microsatellite instability is associated with genetic alteration but not with low levels of expression of the human mismatch repair proteins hMSH2 and hMLH1. Eur J Cancer. 2000; 36:925–931. PMID:
10785599.

134. Lee YY, Kang SH, Seo JY, Jung CW, Lee KU, Choe KJ, et al. Alterations of p16INK4A and p15INK4B genes in gastric carcinomas. Cancer. 1997; 80:1889–1896. PMID:
9366289.

135. Guilford PJ, Hopkins JB, Grady WM, Markowitz SD, Willis J, Lynch H, et al. E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat. 1999; 14:249–255. PMID:
10477433.

136. Sakakura C, Hagiwara A, Nakanishi M, Shimomura K, Takagi T, Yasuoka R, et al. Differential gene expression profiles of gastric cancer cells established from primary tumour and malignant ascites. Br J Cancer. 2002; 87:1153–1161. PMID:
12402156.

137. Sakakura C, Simomura K, Shuichi K, Nakase Y, Fukuda K, Takagi T, et al. [Screening of novel biomarkers for the detection of intraperitoneally disseminated cancer cells using human cDNA microarray]. Gan To Kagaku Ryoho. 2002; 29:2271–2274. PMID:
12484052.
138. Lee HS, Park MH, Yang SJ, Jung HY, Byun SS, Lee DS, et al. Gene expression analysis in human gastric cancer cell line treated with trichostatin A and S-Adenosyl-L-homocysteine using cDNA microarray. Biol Pharm Bull. 2004; 27:1497–1503. PMID:
15467184.

139. Shin JH, Chung J, Kim HO, Kim YH, Hur YM, Rhim JH, et al. Detection of cancer cells in peripheral blood of stomach cancer patients using RT-PCR amplification of tumour-specific mRNAs. Aliment Pharmacol Ther. 2002; 16(Suppl 2):137–144. PMID:
11966534.

140. Shin JY, Kim HS, Park J, Park JB, Lee JY. Mechanism for inactivation of the KIP family cyclin-dependent kinase inhibitor genes in gastric cancer cells. Cancer Res. 2000; 60:262–265. PMID:
10667572.
141. Jeong YW, Kim KS, Oh JY, Park JC, Baek WK, Suh SI, et al. Exogenous wild-type p16INK4A gene induces delayed cell proliferation and promotes chemosensitivity through decreased pRB and increased E2F-1 expressions. Int J Mol Med. 2003; 12:61–65. PMID:
12792810.

142. Choi CH. Cloning and functional study of a novel human metallothionein-I isoform induced by paraquat. Biochem Biophys Res Commun. 2003; 304:236–240. PMID:
12711304.

143. Han SH, Jeon JH, Ju HR, Jung U, Kim KY, Yoo HS, et al. VDUP1 upregulated by TGF-beta1 and 1,25-dihydorxyvitamin D3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene. 2003; 22:4035–4046. PMID:
12821938.
144. Park BK, Moon HR, Yu JR, Kook J, Chai JY, Lee SH. [Comparative susceptibility of different cell lines for culture of Toxoplasma gondii in vitro]. Korean J Parasitol. 1993; 31:215–222. PMID:
8241080.

145. Leung WK, Kim JJ, Wu L, Sepulveda JL, Sepulveda AR. Identification of a second MutL DNA mismatch repair complex (hPMS1 and hMLH1) in human epithelial cells. J Biol Chem. 2000; 275:15728–15732. PMID:
10748105.

146. Yamamoto H, Itoh F, Fukushima H, Hinoda Y, Imai K. Overexpression of cyclooxygenase-2 protein is less frequent in gastric cancers with microsatellite instability. Int J Cancer. 1999; 84:400–403. PMID:
10404093.

147. Bae SI, Park JG, Kim YI, Kim WH. Genetic alterations in gastric cancer cell lines and their original tissues. Int J Cancer. 2000; 87:512–516. PMID:
10918190.

148. Woo DK, Kim HS, Lee HS, Kang YH, Yang HK, Kim WH. Altered expression and mutation of beta-catenin gene in gastric carcinomas and cell lines. Int J Cancer. 2001; 95:108–113. PMID:
11241321.
149. Oh ST, Seo JS, Moon UY, Kang KH, Shin DJ, Yoon SK, et al. A naturally derived gastric cancer cell line shows latency I Epstein-Barr virus infection closely resembling EBV-associated gastric cancer. Virology. 2004; 320:330–336. PMID:
15016554.

150. Han SU, Kim HT, Seong do H, Kim YS, Park YS, Bang YJ, et al. Loss of the Smad3 expression increases susceptibility to tumorigenicity in human gastric cancer. Oncogene. 2004; 23:1333–1341. PMID:
14647420.

151. Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002; 109:113–124. PMID:
11955451.

152. Kang MS, Lee HJ, Lee JH, Ku JL, Lee KP, Kelley MJ, et al. Mutation of p53 gene in hepatocellular carcinoma cell lines with HBX DNA. Int J Cancer. 1996; 67:898–902. PMID:
8824565.
153. Kim SO, Park JG, Lee YI. Increased expression of the insulin-like growth factor I (IGF-I) receptor gene in hepatocellular carcinoma cell lines: implications of IGF-I receptor gene activation by hepatitis B virus X gene product. Cancer Res. 1996; 56:3831–3836. PMID:
8706031.
154. Cho JW, Jeong YW, Han SW, Park JB, Jang BC, Baek WK, et al. Aberrant p16INK4A RNA transcripts expressed in hepatocellular carcinoma cell lines regulate pRb phosphorylation by binding with CDK4, resulting in delayed cell cycle progression. Liver Int. 2003; 23:194–200. PMID:
12955883.

155. Suh SI, Pyun HY, Cho JW, Baek WK, Park JB, Kwon T, et al. 5-Aza-2'-deoxycytidine leads to down-regulation of aberrant p16INK4A RNA transcripts and restores the functional retinoblastoma protein pathway in hepatocellular carcinoma cell lines. Cancer Lett. 2000; 160:81–88. PMID:
11098088.

156. Huang JZ, Xia SS, Ye QF, Jiang HY, Chen ZH. Effects of p16 gene on biological behaviours in hepatocellular carcinoma cells. World J Gastroenterol. 2003; 9:84–88. PMID:
12508357.
157. Chun E, Lee KY. Bcl-2 and Bcl-xL are important for the induction of paclitaxel resistance in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2004; 315:771–779. PMID:
14975768.

158. Hwang HJ, Kim GJ, Lee GB, Oh JT, Chun YH, Park SH. A comprehensive karyotypic analysis on Korean hepatocellular carcinoma cell lines by cross-species color banding and comparative genomic hybridization. Cancer Genet Cytogenet. 2003; 141:128–137. PMID:
12606130.

159. Scharf JG, Dombrowski F, Ramadori G. The IGF axis and hepatocarcinogenesis. Mol Pathol. 2001; 54:138–144. PMID:
11376124.

160. Shin EC, Ahn JM, Kim CH, Choi Y, Ahn YS, Kim H, et al. IFN-gamma induces cell death in human hepatoma cells through a TRAIL/death receptor-mediated apoptotic pathway. Int J Cancer. 2001; 93:262–268. PMID:
11410875.
161. Shin EC, Shin JS, Park JH, Kim H, Kim SJ. Expression of fas ligand in human hepatoma cell lines: role of hepatitis-B virus X (HBX) in induction of Fas ligand. Int J Cancer. 1999; 82:587–591. PMID:
10404075.

162. Shin EC, Shin WC, Choi Y, Kim H, Park JH, Kim SJ. Effect of interferon-gamma on the susceptibility to Fas (CD95/APO-1)-mediated cell death in human hepatoma cells. Cancer Immunol Immunother. 2001; 50:23–30. PMID:
11315506.
163. Park SS, Eom YW, Kim EH, Lee JH, Min do S, Kim S, et al. Involvement of c-Src kinase in the regulation of TGF-beta1-induced apoptosis. Oncogene. 2004; 23:6272–6281. PMID:
15208664.
164. Baek WK, Kim D, Jung N, Yi YW, Kim JM, Cha SD, et al. Increased expression of cyclin G1 in leiomyoma compared with normal myometrium. Am J Obstet Gynecol. 2003; 188:634–639. PMID:
12634633.

165. Foli A, Benvenuto F, Piccinini G, Bareggi A, Cossarizza A, Lisziewicz J, et al. Direct analysis of mitochondrial toxicity of antiretroviral drugs. Aids. 2001; 15:1687–1694. PMID:
11546944.

166. Lee H, Lee SI, Cho J, Choi SU, Yang SI. Synthesis and in vitro evaluation of 1,8-diazaanthraquinones bearing 3-dialkylaminomethyl or 3-(N-alkyl- or N-aryl)carbamoyloxymethyl substituent. Eur J Med Chem. 2003; 38:695–702. PMID:
12932900.

167. Lee SW, Lee YM, Bae SK, Murakami S, Yun Y, Kim KW. Human hepatitis B virus X protein is a possible mediator of hypoxia-induced angiogenesis in hepatocarcinogenesis. Biochem Biophys Res Commun. 2000; 268:456–461. PMID:
10679226.

168. Ferber MJ, Montoya DP, Yu C, Aderca I, McGee A, Thorland EC, et al. Integrations of the hepatitis B virus (HBV) and human papillomavirus (HPV) into the human telomerase reverse transcriptase (hTERT) gene in liver and cervical cancers. Oncogene. 2003; 22:3813–3820. PMID:
12802289.

169. Moon MS, Lee CJ, Um SJ, Park JS, Yang JM, Hwang ES. Effect of BPV1 E2-mediated inhibition of E6/E7 expression in HPV16-positive cervical carcinoma cells. Gynecol Oncol. 2001; 80:168–175. PMID:
11161855.

170. Baykal A, Rosen D, Zhou C, Liu J, Sahin AA. Telomerase in breast cancer: a critical evaluation. Adv Anat Pathol. 2004; 11:262–268. PMID:
15322492.
171. Zhou C, Smith JL, Liu J. Role of BRCA1 in cellular resistance to paclitaxel and ionizing radiation in an ovarian cancer cell line carrying a defective BRCA1. Oncogene. 2003; 22:2396–2404. PMID:
12717416.

172. Campbell M, Qu S, Wells S, Sugandha H, Jensen RA. An adenoviral vector containing an arg-gly-asp (RGD) motif in the fiber knob enhances protein product levels from transgenes refractory to expression. Cancer Gene Ther. 2003; 10:559–570. PMID:
12833136.

173. Park SH, Park SY, Kim DW, Chun YH. Chromosomal aberrations in ovarian malignant brenner tumor cell line using chromosome painting. Cancer Genet Cytogenet. 2000; 118:151–153. PMID:
10748297.

174. Yu S, Lee M, Shin S, Park J. Apoptosis induced by progesterone in human ovarian cancer cell line SNU-840. J Cell Biochem. 2001; 82:445–451. PMID:
11500921.

175. Chang SH, Kim SH, Lee WK, Kim HJ, Choi SH, Park JH, et al. Cloning and analysis of the Epstein-Barr virus glycoprotein 350 genes. Mol Cells. 1998; 8:585–593. PMID:
9856346.
176. Kim HR, Jeong JA, Park CH, Lee SK, Lee WK, Jang YS. A role for cell cycle proteins in the serum-starvation resistance of Epstein-Barr virus immortalized B lymphocytes. Biochem Cell Biol. 2002; 80:407–413. PMID:
12234093.

177. Lee W, Hwang YH, Lee SK, Subramanian C, Robertson ES. An Epstein-Barr virus isolated from a lymphoblastoid cell line has a 16-kilobase-pair deletion which includes gp350 and the Epstein-Barr virus nuclear antigen 3A. J Virol. 2001; 75:8556–8568. PMID:
11507201.

178. Park CH, Kim HR, Kim J, Jang SH, Lee KY, Chung GH, et al. Latent membrane protein 1 of Epstein-Barr virus plays an important role in the serum starvation resistance of Epstein-Barr virus-immortalized B lymphocytes. J Cell Biochem. 2004; 91:777–785. PMID:
14991769.

179. Koh TY, Park SW, Park KH, Lee SG, Seol JG, Lee DW, et al. Inhibitory effect of p27KIP1 gene transfer on head and neck squamous cell carcinoma cell lines. Head Neck. 2003; 25:44–49. PMID:
12478543.

180. Park SW, Lee SG, Song SH, Heo DS, Park BJ, et al. The effect of nitric oxide on cyclooxygenase-2 (COX-2) overexpression in head and neck cancer cell lines. Int J Cancer. 2003; 107:729–738. PMID:
14566822.

181. Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, et al. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002; 21:6598–6605. PMID:
12242657.

182. Chung PS, Kim HG, Rhee CK, Saxton RE. Anticancer effect of combined intratumor cisplatin injection and interstitial KTP laser therapy on xenografted squamous cell carcinoma. J Clin Laser Med Surg. 2003; 21:23–27. PMID:
12614556.

183. Sung MW, Roh JL, Park BJ, Park SW, Kwon TK, Lee SJ, et al. Bile acid induces cyclo-oxygenase-2 expression in cultured human pharyngeal cells: a possible mechanism of carcinogenesis in the upper aerodigestive tract by laryngopharyngeal reflux. Laryngoscope. 2003; 113:1059–1063. PMID:
12782823.

184. Kang YH, Lee E, Choi MK, Ku JL, Kim SH, Park YG, et al. Role of reactive oxygen species in the induction of apoptosis by alpha-tocopheryl succinate. Int J Cancer. 2004; 112:385. PMID:
15382062.
185. Hansel DE, Rahman A, Hidalgo M, Thuluvath PJ, Lillemoe KD, Shulick R, et al. Identification of novel cellular targets in biliary tract cancers using global gene expression technology. Am J Pathol. 2003; 163:217–229. PMID:
12819026.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download