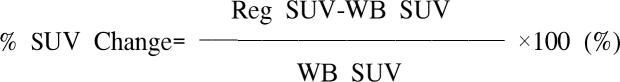1. Hagge RJ, Wong TZ, Coleman RE. Positron emission tomography: brain tumors and lung cancer. Radiol Clin North Am. 2001; 39:871–881. PMID:
11587059.
2. Seltzer MA, Yap CS, Silverman DH, Meta J, Schiepers C, Phelps ME, et al. The impact of PET on management of lung cancer: the referring physician's perspective. J Nucl Med. 2002; 43:752–756. PMID:
12050318.
3. Hoh CK, Hawkins RA, Glaspy JA, Dahlbom M, Tse NY, Hoffman EJ, et al. Cancer detection with whole-body PET using 2-[
18F]Fluoro-2-deoxy-D-glucose. J Comput Assist Tomogr. 1993; 17:582–589. PMID:
8331230.
4. Lowe VJ, DeLong DM, Hoffman JM, Coleman RE. Optimum scanning protocol for FDG-PET evaluation of pulmonary malignancy. J Nucl Med. 1995; 36:883–887. PMID:
7738668.
5. Fischman AJ, Alpert NM. FDG-PET in oncology: there's more to it than looking at pictures. J Nucl Med. 1993; 34:6–11. PMID:
8418272.
6. Hamberg LM, Hunter GJ, Alpert NM, Choi NC, Babich JW, Fischman AJ. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med. 1994; 35:1308–1312. PMID:
8046485.
7. Lodge MA, Lucas JD, Marsden PK, Cronin BF, ODoherty MJ, Smith MA. A PET study of
18FDG uptake in soft tissue masses. Eur J Nucl Med. 1999; 26:22–30. PMID:
9933658.
8. Boerner AR, Weckesser M, Herzog H, Schmitz T, Audretsch W, Nitz U, et al. Optimal scan time for fluorine-18 fluorodeoxyglucose positron emission tomography in breast cancer. Eur J Nucl Med. 1999; 26:226–230. PMID:
10079312.

9. Hustinx R, Smith RJ, Benard F, Rosenthal DI, Machtay M, Farber LA, et al. Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J Nucl Med. 1999; 26:1345–1348. PMID:
10541835.

10. Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, et al. Dual time point
18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001; 42:1412–1417. PMID:
11535734.
11. Matthies A, Hickeson M, Cuchiara A, Alavi A. Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med. 2002; 43:871–875. PMID:
12097455.
12. Kubota R, Kubota K, Yamada S, Tada M, Ido T, Tamahashi N. Microautoradiographic study for the differentiation of intratumoral macrophages, granulation tissues and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose uptake. J Nucl Med. 1994; 35:104–112. PMID:
8271030.
13. Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997; 111:1718–1723. PMID:
9187199.

14. National Electrical manufacturers Association. NEMA Standards Publication NU2-1994. 1994. Washington DC: National Electrical Manufacturers Association.
15. Reivich M, Alavi A, Wolf A, Fowler J, Russell J, Arnett C, et al. Glucose metabolic rate kinetic model parameter determination in humans: the lumped constants and rate constants for [
18F]fluorodeoxyglucose and [
11C]deoxyglucose. J Cereb Blood Flow Metab. 1985; 5:179–192. PMID:
3988820.
16. Nolop KB, Rhodes CG, Brudin LH, Beaney RP, Krausz T, Jones T, et al. Glucose utilization in vivo by human pulmonary neoplasms. Cancer. 1987; 60:2682–2689. PMID:
3499969.
17. Kubota K, Itoh M, Ozaki K, Ono S, Tashiro M, Yamaguchi K, et al. Advantage of delayed whole-body FDG-PET imaging for tumour detection. Eur J Nucl Med. 2001; 28:696–703. PMID:
11440029.

18. Yamada S, Kubota K, Kubota R, Ido T, Tamahashi N. High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue. J Nucl Med. 1995; 36:1301–1306. PMID:
7790960.
19. Demura Y, Tsuchida T, Ishizaki T, Mizuno S, Totani Y, Ameshima S, et al.
18F-FDG accumulation with PET for differentiation between benign and malignant lesions in the thorax. J Nucl Med. 2003; 44:540–548. PMID:
12679397.