Abstract
Colorectal cancer appears to have rapidly increased over the past two decades in Korea. Environmental factors, characterized by a western life style, seem to be closely related to the increased risk of colorectal cancer. Higher intakes of meat, a lower vegetable intake, a lack of physical activity, obesity, and alcohol drinking have been suggested to be risk factors for colorectal cancer in the numerous epidemiologic studies. Several specific associations have also been observed between genetic polymorphisms and colorectal cancer. Moreover, it has been postulated that environmental factors and a genetic predisposition work in concert in colorectal cancer development. A stronger association between red meat intake and colorectal cancer among those with rapid acetylators at either the NAT1 or NAT2 locus was reported, particularly for colorectal cancer associated with K-ras mutations. The protective effect of the homozygous variant TT form of the MTHFR genotype on the risk of colon cancer seems to be modified by the level of methyl diets, i.e., by folate, which has a protective effect, or conversely by alcohol. The insulin-related pathway, which possibly explains at a mechanistic level the effect of physical activity and obesity on colon cancer, appears to be a common denominator in colon cancer and in other metabolic disorders, such as diabetes mellitus and dyslipidemia. Hyperinsulinemia has been proposed as an explanation for the association between a Western lifestyle and colon cancer risk. Further studies, that incorporate both genetic and environmental factors, are needed to fully explain and identify the underlying pathway of colorectal carcinogenesis.
Colorectal cancer is the fourth most common cancer and accounts for 8.5% of all incident cases worldwide (1). Age-standardized incidence rates, however, vary by 20-fold across countries. The highest rates occur in Western societies, such as, in North America, Western Europe, Australia, and New Zealand (30~50 per 100,000), while rates were low in most Asian and African countries (less than 10 per 100,000) in the late 1980s. However, recently incidences have increased in some Asian countries like Japan (2). According to the recent incidence statisties (3), the highest incidence was reported in Hiroshima, Japan (86.7 per 100,000 in men). The incidence of colorectal cancer have also increased rapidly over the past two decades in Korea, which was previously known as low risk area. Age-standardized mortality rates in Korea increased from 2.4 in 100,000 to 10.2 in 100,000 for men and from 1.8 in 100,000 to 6.0 in 100,000 for women between 1983 and 2000 (4). Moreover, the increased mortality due to colorectal cancer over the same period appears to be steeper than that of lung cancer in men and women equally (Fig. 1). Based on the Centra Cancer Registry Program (CCRP), a hospital-based cancer registry operated by the Korean government since 1980, the proportion of colorectal cancer case among total registered cancers increased in men and women from 5.8% and 5.8% in 1983 to 11.6% and 10.7% in 2002, respectively (5).
Studies on migrants populations showed that those moving from low- to high- risk countries acquired an elevated risk of large bowel cancer even in the first-generation (6,7). It was proposed that environmental factors, characterized by a western life style, are closely related to the risk of colorectal cancer (8). Numerous studies have suggested that a higher intake of red meat, and possibly in association with the cooking process, may increase risk of colorectal cancer. In addition, it has been consistently reported that physical activity and obesity are associated with colorectal cancer risk. Recently, methyl diets, such as, folate and alcohol intake, have also be related to colon cancer risk. And, several studies have noted a higher risk of colon cancer among smokers, especially among those with long smoking histories.
There appears to be a familial aggregation in colorectal cancer, which suggests that a genetic predisposition may be important in the etiology of the disease. A number of associations have also been observed between specific genetic polymorphisms and colorectal cancer. It was argued that most colorectal cancers are neither purely genetic nor purely environmental (9). Environmental factors, both dietary and other environmental factors, appear to interact with genetic factors in the development of colorectal cancer.
In this review, we present how some established environmental factors interact with genetic factors to modify the risk of colorectal cancer.
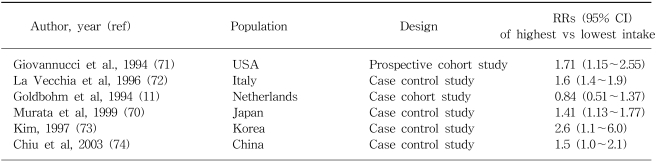
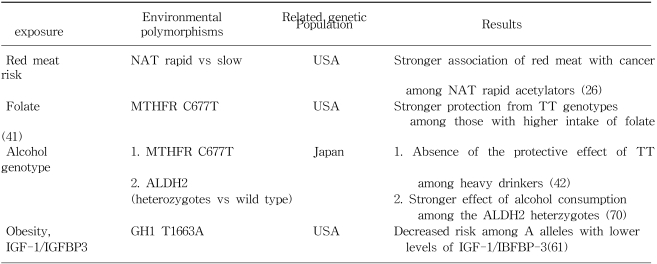
Rate of colon cancer was reported to be strongly correlated with the national per capita consumption of animal fat in one large scale international ecologic study (10). The majority of epidemiologic studies have shown a positive association with intake of red meat (8), with some exceptions (11). The effect estimates between red meat and colon cancer for some selected populations are shown in Table 1. Based on one recent meta-analysis (12), the average relative risks (RRs) and 95% confidence intervals (CI) for the highest vs lowest quantile of consumption of red meat were 1.35 (95% CI: 1.21~1.51) and of processed meat, 1.31 (95% CI: 1.13~1.51). Total meat consumption was not significantly associated with colorectal cancer risk. In terms of the measurable nutrients in meat, it is not clear which are truly associated with colorectal cancer risk. The Nurses??Health Study, prospective cohort study consisting of 120,000 registered nurses, showed that women in the highest quartile of red meat intake, compared with those in the lowest quartile, showed a 2.75-fold increase in colon cancer risk, even after adjusting for fat intake (13). Giovannucci and Gordin concluded that the association with red meat consumption does not appear to be mediated by its lipid content (14). Sugimura and Sato proposed that heterocyclic amines (HCAs), which are potent carcinogens, are associated with the risk of colon cancer (15). HCAs are formed by heating creatinine with amino acids, which occurs when meat is fried, grilled, or broiled at high temperature (16). Some studies have suggested, but not all (17), that risk of colon cancer (18,19) or adenoma (20) may be increased among those who prefer meat with browned surface. These observations raised the question as to whether enzymatic variabilities in the metabolism of heterocyclic amines, such as N-acetyltransferase (NAT), might influence the risk of colorectal neoplasia (21). Although no independent association with NAT1 (22) or NAT2 (23) genotypes were found, some studies (24~26) reported that rapid acetylators at either the NAT1 or NAT2 locus show a stronger association between red meat intake and colorectal cancer, suggesting that genetic polymorphisms may interact with meat consumption. Furthermore, the prevalence of the K-ras mutation, which is observed in 40 to 50% of colorectal neoplasm (27), has been closely linked to the rapid acetylator phenotype of the NAT2 enzyme. Therefore, NAT2 may determine genetic susceptibility to adenomas, carcinomas, and specific gene mutations, and may thus modify the association between meat consumption and colorectal cancer.
Higher intakes of vegetables have been reported to be associated with a reduced risk of colorectal cancer (28,29). These effects of vegetables have been particularly consistent for raw vegetables, green vegetables, and cruciferous vegetables (8). It was suggested that the effect of vegetables on the risk of colon cancer appears to act through other pathways than dietary fiber (30). Folate, a water-soluble B vitamins, was suggested to be responsible for the beneficial effect of vegetables.
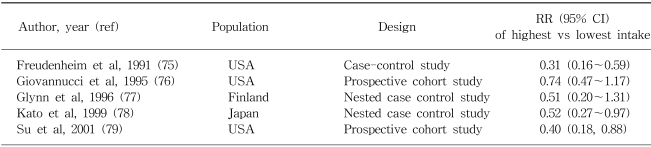
Epidemiologic studies on the effect of folate in colorectal cancer are limited. Lashner et al (31) first made a relevant observation in ulcerative colitis patients in a case-control study, they found that individuals who had not been regularly taking folate supplements had a rate of colonic neoplasms approximately 2.5-fold greater than those who had been taking supplements. Since then, fourteen studies have examined the association between folate intake and colorectal cancer risk and five studies have evaluated folate intake in relation to the risk of adenomatous polyps, the precusor lesions of colorectal cancer (32). However, results from case-control studies have been inconsistent. Inverse associations between higher intakes of folate and colorectal cancer risk have been more consistently observed in prospective studies. The effect estimates between folate and colon cancer for some selected populations are shown in Table 2.
Several mechanisms have been proposed for the effect of folate. First, folate is critical for the synthesis of S-adenosylmethionine (SAM), a compound that serves as an essential methyl donor for over 100 biochemical reactions, including the methylation of DNA (33). Consequently diminished folate intake might favor global and/or regional DNA hypomethylation, which appears to be an early, and consistent event in carcinogenesis (34). Second, the misincorporation of uracil into human DNA might be favored when thymidylate availability is restricted, i.e., due to folate deficiency (35,36), which is related to an increased frequency of chromosome cleavage (37). Finally the secondary depletion of choline due to folate deficiency might activate protein kinase C (PKC) signaling, which is related with mitogenesis and to the enhanced expression of the c-myc proto-oncogene (38). PKC activation has been reported to occur early in the development of chemically induced colonic neoplasia (39), and in human colorectal cancers (40).
Methylenetetrahydrofolate reductase (MTHFR) is a key regulator of the folate metabolism. MTHFR converts 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is the major form of folate in the blood and the primary methyl donor in the formation of methionine. A common genetic polymorphism of MTHFR (C677T, alanine-to-valine) has recently drawn much interest in the etiology of colorectal cancer. Individuals with a homozygous variant of the MTHFR (TT genotype) were found to have a reduced risk of colorectal cancer in several studies in the United States (41~44). This effect of the homozygous mutation of MTHFR was hypothesized to be related to the quantitative balance of 5, 10 methylenetetrahydrofolate and 5-methyltetrahydrofolate (41). However, in a study conducted in the United States (41,42), a protective association between the TT genotype and colorectal cancer was pronounced when folate or methionine intake was high and was disappeared when alcohol intake was high, suggesting that the effect of MTHFR genetic polymorphism is modified by the levels of dietary factors related with methyl metabolism. Contrary to these protective findings with TT genotype of MTHFR, an increased risk of CRC with the TT genotype was reported in some case-control studies conducted in Australia, especially among the elderly (45), and in Mexico (46). A similar positive association was also observed in a case-control study conducted in Korea (unpublished data). These findings raise the question as to whether the effect of MTHFR may differ according to the intake patterns of these dietary factors across countries.
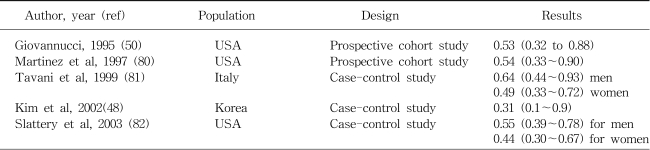
The relation between physical activity and a reduced risk of colon cancer is among the most consistent findings in the epidemiologic literature. Based on one recent review (47), prospective and retrospective studies, upon occupational, leisure, and total activities, support an inverse association between physical activity and the risk of colon, but not of rectal cancer. Physically active men and women show a 30~40% dose-dependent reduction in the risk of colon cancer, compared with inactive individuals. The similar, but stronger risk reduction was also reported for the heavy leisure time physical activity among the elderly in a case-control study conducted in Korea (48). The effect estimates between physical activity and colon cancer for some selected populations are shown in Table 3. Obesity appears to be associated with an increased risk of colon cancer. For example, body mass index (BMI) was positively associated with colon cancer and large (≥ 1 cm) adenoma but not with small adenoma (49). When physical activity and obesity were assessed jointly, the highest risk of colon cancer was found among those who were both physically inactive and had high BMI levels (50,51).
Several biologic mechanisms have been proposed to explain the inverse association between physical activity, body mass, and colon cancer. One favored hypothesis concerns the relation between these factors and insulin. Hyperinsulinemia is related to physical inactivity and a high BMI (52). Moreover, insulin is mitogenic for normal and neoplastic colonic epithelial cells, and has been shown to be a colon tumor promoter in animal model (53). In addition, high insulin levels have been related prospectively to colon cancer risk (54). Hu et al (55) reported an increased incidence of colorectal cancer among diabetes mellitus patients in the Nurses' Health Study. It appears that insulin acts as a common denominator in colon cancer and other metabolic disorders, such as diabetes mellitus and dyslipidemia. Actually, hyperinsulinemia has been proposed to underlie the association between a Western lifestyle and colon cancer risk (56).
Insulin-like growth factor (IGF) axis influences cellular proliferation and apoptosis. Insulin-like growth factor-I (IGF-I) is a potent mitogen for normal and neoplastic cells, whereas IGF-binding protein-3 (IGFBP-3) has been found to inhibit cell growth in many experimental systems. Recently it was reported that colorectal cancer is positively related to plasma insulin-like growth factor I (IGF-I), and inversely related to insulin-like growth factor binding protein 3 (IGFBP-3) by several prospective studies (57~59). Further Ma et al (60) reported that elevated insulin production, as reflected by an elevated concentrations of plasma C-peptide, appeared to predict the risk of colorectal cancer development, independently of BMI, factors related to insulin resistance, or levels of IGF-I and IGFBP-3 in a nested case-control study. Recently a T-to-A polymorphism in the growth hormone (GH)1 at position 1663, which is supposedly conferring lower levels of IGF-1, was found to be associated with a decreased risk of colorectal cancer in a case-control study conducted in Hawaii (61). These associations were, however, observed in Caucasians and Native Hawaiians but not in Japanese.
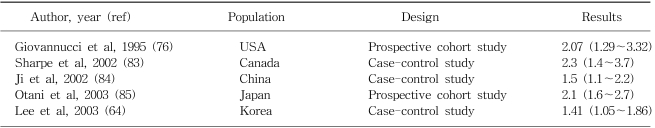
Alcohol intake has been related to an increased risk of colorectal cancer although the increase is relatively small (8). A meta-analysis of 5 cohort studies and 22 case-control studies published from 1966 to 1989 showed a weak positive association (62). In a recent pooled analysis of 8 prospective cohort studies from Western countries, an 16% increase in the risk of colorectal cancer was reported among those consuming 30g or more of alcohol per day, with no appreciable differences shown between colon and rectal cancers in this regard (63). Similar association was also reported in a case-control study conducted in Korea (64). No convincing evidence suggests that different sources of alcohol present different risk (65). The association is likely to be related to total ethanol intake, irrespective of the type of drink. The effect estimates between alcohol consumption and colon cancer for some selected populations are shown in Table 4.
Several mechanisms are conceivable with respect to the increased risk of colorectal cancer associated with alcohol intake. In rats fed a diet with a normal folate content, alcohol administration increased intracolonic acetaldehyde levels and significantly reduced colonic mucosal folate levels (66), possibly due to cleavage of folate by acetaldehyde (67). Elevated alcohol intake may also be related with delayed DNA repair, the activation of liver procarcinogen by the induction of cytochrome p-450 enzymes (68), or a change in bile acid composition (69). The beneficial effect of the MTHFR TT genotype on the risk of CRC was abolished in those with high alcohol intakes (41,42), suggesting a possible interaction between genotypes and folate metabolism. Further, the association with colon cancer due to alcohol consumption appeared to be pronounced among the heterozygotes of Aldehyde dehydrogenase 2 (ALDH2), which encodes a mitochondrial enzyme responsible for the oxidation of acetaldehyde generated in alcohol metabolism, compared with the wild type (70).
Based on current evidences, there appears to be more than one pathway to the development of colorectal cancer. Environmental factors, such as obesity, physical activity, folate, red meat consumption, and alcohol drinking, appear to interact with specific genetic polymorphisms to modify the risk of colon cancer (summarized in Table 5). Thus further studies incorporating genetic and environmental factors are needed to fully explain and characterize the nature of the pathway that underlies colorectal carcinogenesis.
References
1. Cancer Incidence in Five Continents. 1997. Lyon: International Agency for Research on Cancer.
2. Tamura K, Ishiguro S, Munakata A, Yoshida Y, Nakaji S, Sugawara K. Annual changes in colorectal carcinoma incidence in Japan. Analysis of survey data on incidence in Aomori Prefecture. Cancer. 1996; 78:1187–1194. PMID: 8826939.

3. Parkin DM, editor. Cancer Incidence in Five Continents. 2002. Volume VIII:International Agency for Research on Cancer;IARC scientific publication; 155.
4. Causes of death. 1983-2000. Korea: National Bureau of Statistics.
5. One year's report for cancer registry programme in Republic of Korea. 1983-2002. Korea: Ministry of Health and Welfare.
6. Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968; 40:43–68. PMID: 5635018.
7. McMichael AJ, Giles GG. Cancer in migrants to Australia: extending the descriptive epidemiological data. Cancer Res. 1988; 48:751–756. PMID: 3335035.
8. Potter JD, Slattery ML, Bostick RM, Gapstur SM. Colon cancer: a review of the epidemiology. Epidemiol Rev. 1993; 15:499–545. PMID: 8174669.

9. Mucci LA, Wedren S, Tamimi RM, Trichopoulos D, Adami HO. The role of gene-environment interaction in the aetiology of human cancer: examples from cancers of the large bowel, lung and breast. J Intern Med. 2001; 249:477–493. PMID: 11422654.

10. Rose DP, Boyar AP, Wynder EL. International comparisons of mortality rates for cancer of the breast, ovary, prostate, and colon, and per capita food consumption. Cancer. 1986; 58:2363–2371. PMID: 3768832.

11. Goldbohm RA, van den Brandt PA, van't Veer P, Brants HAM, Dorant E, Sturmans F, Hermus RJJ. A prospective cohort study on the relation between meat consumption and the risk of colon cancer. Cancer Res. 1994; 54:718–723. PMID: 8306333.
12. Norat T, Lukanova A, Ferrari P, Riboli E. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer. 2002; 98:241–256. PMID: 11857415.

13. Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990; 323:1664–1672. PMID: 2172820.

14. Giovannucci E, Goldin B. The role of fat, fatty acids, and total energy intake in the etiology of human colon cancer. Am J Clin Nutr. 1997; 66:1564S–1571S. PMID: 9394716.

15. Sugimura T, Sato S. Mutagens-carcinogens in foods. Cancer Res. 1983; 43:2415S–2421S. PMID: 6682010.
16. Wakabayashi K, Nagao M, Esumi H, Sugimura T. Foodderived mutagens and carcinogens. Cancer Res. 1992; 52:2092s–2098s. PMID: 1544146.
17. Augustsson K, Skog K, Jagerstad M, Dickman PW, Steineck G. Dietary heterocyclic amines and cancer of the colon, rectum, bladder, and kidney: a population-based study [see comments]. Lancet. 1999; 353:703–707. PMID: 10073512.
18. Gerhardsson de Verdier M, Hagman U, Peters RK, Steineck G, Overik E. Meat, cooking methods and colorectal cancer: A case-referent study in Stockholm. Int J Cancer. 1991; 49:520–525. PMID: 1917152.
19. Lee HP, Gourley L, Duffy SW, Esteve J, Lee J, Day NE. Colorectal cancer and diet in an Asian population--A case-control study among Singapore Chinese. Int J Cancer. 1989; 43:1007–1016. PMID: 2731998.

20. Sinha R, Chow WH, Kulldorff M, Denobile J, Butler J, Garcia-Closas M, Weil R, Hoover RN, Rothman N. Well-done, grilled red meat increases the risk of colorectal adenomas. Cancer Res. 1999; 59:4320–4324. PMID: 10485479.
21. Kadlubar FF, Butler MA, Kaderlik KR, Chou HC, Lang NP. Polymorphisms for aromatic amine metabolism in humans: relevance for human carcinogenesis. Environ Health Perspect. 1992; 98:69–74. PMID: 1486865.

22. Lin HJ, Probst-Hensch NM, Hughes NC, Sakamoto GT, Louie AD, Kau IH, Lin BK, Lee DB, Lin J, Frankl HD, Lee ER, Hardy S, Grant DM, Haile RW. Variants of N-acetyltransferase NAT1 and a case-control study of colorectal adenomas. Pharmacogenetics. 1998; 8:269–281. PMID: 9682272.

23. Welfare MR, Cooper J, Bassendine MF, Daly AK. Relationship between acetylator status, smoking, and diet and colorectal cancer risk in the north-east of England. Carcinogenesis. 1997; 18:1351–1354. PMID: 9230278.

24. Bell DA, Badawi AF, Lang NP, Ilett KF, Kadlubar FF, Hirvonen A. Polymorphism in the N-acetyltransferase 1 (NAT1) polyadenylation signal: association of NAT1*10 allele with higher N-acetylation activity in bladder and colon tissue. Cancer Research. 1995; 55:5226–5229. PMID: 7585580.
25. Roberts-Thomson IC, Ryan P, Khoo KK, Hart WJ, McMichael AJ, Butler RN. Diet, acetylator phenotype, and risk of colorectal neoplasia. Lancet. 1996; 347:1372–1374. PMID: 8637343.

26. Chen J, Stampfer MJ, Hough HL, Garcia-Closas M, Willett WC, Hennekens CH, Kelsey KT, Hunter DJ. A prospective study of N-acetyltransferse genotype, red meat intake, and risk of colorectal cancer. Cancer Res. 1998; 58:3307–3311. PMID: 9699660.
27. Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AMM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988; 319:525–532. PMID: 2841597.

28. Trock B, Lanza E, Greenwald P. Dietary fiber, vegetables, and colon cancer: critical review and meta-analyses of the epidemiologic evidence. J Natl Cancer Inst. 1990; 82:650–661. PMID: 2157027.

29. Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996; 96:1027–1039. PMID: 8841165.
30. Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Stampfer MJ, Rosner B, Speizer FE, Willett WC. Dietary fiber and the risk of colorectal cancer and adenoma in women. N Engl J Med. 1999; 340:169–176. PMID: 9895396.

31. Lashner BA, Heidenreich PA, Su GL, Kane SV, Hanauer SB. Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis: a case-control study. Gastroenterology. 1989; 97:255–259. PMID: 2568304.
32. Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. 2002; 132:2350S–2355S. PMID: 12163691.

33. Kim YI. Folate and carcinogenesis: Evidence, mechanisms, and implications. J Nutr Biochem. 1999; 10:66–88. PMID: 15539274.

34. Zingg JM, Jones PA. Genetic and epigenetic aspects of DNA methylation on genome expression, evolution, mutation and carcinogenesis. Carcinogenesis. 1997; 18:869–882. PMID: 9163670.

35. Sedwick WD, Kutler M, Brown OE. Antifolate-induced misincorporation of deoxyuridine monophosphate into DNA: inhibition of high molecular weight DNA synthesis in human lymphoblastoid cells. Proc Natl Acad Sci USA. 1981; 78:917–921. PMID: 6940156.

36. Eto I, Krumdieck CL. Role of vitamin B12 and folate deficiencies in carcinogenesis. Adv Exp Med Biol. 1986; 206:313–330. PMID: 3591525.

37. Blount BC, Mack MM, Wehr CM, MacGregor JT, Hiatt RA, Wang G, Wickramasinghe SN, Everson RB, Ames BN. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci USA. 1997; 94:3290–3295. PMID: 9096386.

38. Rozengurt E. Early signals in the mitogenic response. Science. 1986; 234:161–166. PMID: 3018928.

39. Baum CL, Wali RK, Sitrin MD, Bolt MJ, Brasitus TA. 1, 2-Dimethylhydrazine-induced alterations in protein kinase C activity in the rat preneoplastic colon. Cancer Res. 1990; 50:3915–3920. PMID: 2162248.
40. Guillem JG, O'Brian CA, Fitzer CJ, Forde KA, LoGerfo P, Treat M, Weinstein IB. Altered levels of protein kinase C and Ca2+-dependent protein kinases in human colon carcinomas. Cancer Res. 1987; 47:2036–2039. PMID: 3828992.
41. Chen J, Giovannucci E, Kelsey K, Rimm EB, Stampfer MJ, Colditz GA, Spiegelman D, Willett WC, Hunter DJ. A methylenetetrahydrofolate reductase polymorphism and the risk of colorectal cancer. Cancer Res. 1996; 56:4862–4864. PMID: 8895734.
42. Ma J, Stampfer MJ, Giovannucci E, Artigas C, Hunter DJ, Fuchs C, Willett WC, Selhub J, Hennekens CH, Rozen R. Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res. 1997; 57:1098–1102. PMID: 9067278.
43. Slattery ML, Potter JD, Samowitz W, Schaffer D, Leppert M. Methylenetetrahydrofolate reductase, diet, and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 1999; 8:513–518. PMID: 10385141.
44. Le Marchand L, Donlon T, Hankin JH, Kolonel LN, Wilkens LR, Seifried A. B-vitamin intake, metabolic genes, and colorectal cancer risk (United States). Cancer Causes Control. 2002; 13:239–248. PMID: 12020105.
45. Shannon B, Gnanasampanthan S, Beilby J, Iacopetta B. A polymorphism in the methylenetetrahydrofolate reductase gene predisposes to colorectal cancers with microsatellite instability. Gut. 2002; 50:520–524. PMID: 11889073.

46. Delgado-Enciso I, Martinez-Garza SG, Rojas-Martinez A, Ortiz-Lopez R, Bosques-Padilla F, Calderon-Garciduenas AL, Zarate-Gomez M, Barrera-Saldana HA. [677T mutation of the MTHFR gene in adenomas and colorectal cancer in a population sample from the Northeastern Mexico. Preliminary results]. Rev Gastroenterol Mex. 2001; 66:32–37. PMID: 11464627.
47. Lee IM. Physical activity and cancer prevention--data from epidemiologic studies. Med Sci Sports Exerc. 2003; 35:1823–1827. PMID: 14600545.

48. Kim DH, Ahn YO, Lee BW, Whang DY, Lee HJ. A Case-Control Study on the Association between Physical Activity and Colorectal Cancer Risk in Korea. J Kor Asso Ca Prev. 2002; 7:116–126.
49. Giovannucci E. Diet, body weight, and colorectal cancer: a summary of the epidemiologic evidence. J Womens Health (Larchmt). 2003; 12:173–182. PMID: 12737716.

50. Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995; 122:327–334. PMID: 7847643.

51. Slattery ML, Potter J, Caan B, Edwards S, Coates A, Ma K-N, Berry TD. Energy balance and colon cancer--beyond physical activity. Cancer Res. 1997; 57:75–80. PMID: 8988044.
52. Westerlind KC. Physical activity and cancer prevention--mechanisms. Med Sci Sports Exerc. 2003; 35:1834–1840. PMID: 14600547.

53. Tran TT, Medline A, Bruce R. Insulin promotion of colon tumors in rats. Cancer Epidemiol Biomarkers Prev. 1996; 5:1013–1015. PMID: 8959325.
54. Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, Dobs A, Savage PJ. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999; 91:1147–1154. PMID: 10393723.

55. Hu FB, Manson JE, Liu S, Hunter D, Colditz GA, Michels KB, Speixzer FE, Giovannucci E. Prospective study of adult onset diabetes mellitus (Type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999; 91:542–547. PMID: 10088625.

57. Ma J, Pollak MN, Giovannucci E, Chan JM, Tao T, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-1 and IGF-binding protein-3. J Natl Cancer Inst. 1999; 91:620–625. PMID: 10203281.
58. Giovannucci E, Pollak MN, Platz EA, Willett WC, Stampfer MJ, Majeed N, Colditz GA, Speizer FE, Hankinson SE. Plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal cancer and adenoma in women [abstract #1399]. 1999. In : American Association for Cancer Research 90th Annual Meeting Proceedings; Philadelphia, PA: –211.

59. Palmqvist R, Hallmans G, Rinaldi S, Biessy C, Stenling R, Riboli E, Kaaks R. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut. 2002; 50:642–646. PMID: 11950809.

60. Ma J, Giovannucci E, Pollak M, Leavitt A, Tao Y, Gaziano JM, Stampfer MJ. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst. 2004; 96:546–553. PMID: 15069117.

61. Le Marchand L, Donlon T, Seifried A, Kaaks R, Rinaldi S, Wilkens LR. Association of a common polymorphism in the human GH1 gene with colorectal neoplasia. J Natl Cancer Inst. 2002; 94:454–460. PMID: 11904318.

62. Longnecker MP, Orza MJ, Adams ME, Vioque J, Chalmers TC. A meta-analysis of alcoholic beverage consumption in relation to risk of colorectal cancer. Cancer Causes Control. 1990; 1:59–68. PMID: 2151680.

63. Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Holmberg L, Kim DH, Malila N, Miller AB, Pietinen P, Rohan TE, Sellers TA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004; 140:603–613. PMID: 15096331.
64. Lee HJ, Ahn YO, Lee BW, Whang DY, Kono S, Kim DH. The effect of alcohol drinking on the subsite-specific risk of colorectal cancer. 2002. In : 54th Annual Meeting of Society of Preventive Medicine; Sokcho.
65. Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999; 91:916–932. PMID: 10359544.

66. Homann N, Tillonen J, Salaspuro M. Microbially produced acetaldehyde from ethanol may increase the risk of colon cancer via folate deficiency. Int J Cancer. 2000; 86:169–173. PMID: 10738242.

67. Salaspuro M. Bacteriocolonic pathway for ethanol oxidation: characteristics and implications. Ann Med. 1996; 28:195–200. PMID: 8811162.

68. Seitz HK, Poschl G, Simanowski UA. Alcohol and cancer. Recent Dev Alcohol. 1998; 14:67–95. PMID: 9751943.

69. Kune GA, Vitetta L. Alcohol consumption and the etiology of colorectal cancer: a review of the scientific evidence from 1957 to 1991. Nutr Cancer. 1992; 18:97–111. PMID: 1437657.

70. Murata M, Tagawa M, Watanabe S, Kimura H, Takeshita T, Morimoto K. Genotype difference of aldehyde dehydrogenase 2 gene in alcohol drinkers influences the incidence of Japanese colorectal cancer patients. Jpn J Cancer Res. 1999; 90:711–719. PMID: 10470282.

71. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994; 54:2390–2397. PMID: 8162586.
72. La Vecchia C, Ferraroni M, Mezzetti M, Enard L, Negri E, Franceschi S, Decarli A. Attributable risks for colorectal cancer in northern Italy. Int J Cancer. 1996; 66:60–64. PMID: 8608968.

73. Kim DH, Ahn YO, Park BJ, Shin MH, Park JG, Lee BH, Whang DY. Relation of meat intake and body mass index to the risk of colorectal cancer. 1997. In : Federation Meeting of Korean Basic Medical Society 5th Annual Meeting Proceedings; Seoul, Korea: –160.
74. Chiu BC, Ji BT, Dai Q, Gridley G, McLaughlin JK, Gao YT, Fraumeni JF Jr, Chow WH. Dietary factors and risk of colon cancer in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2003; 12:201–208. PMID: 12646508.
75. Freudenheim JL, Graham S, Marshall JR, Haughey BP, Cholewinski S, Wilkinson G. Folate intake and carcinogenesis of the colon and rectum. Int J Epidemiol. 1991; 20:368–374. PMID: 1917236.

76. Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Willett WC. Alcohol, low-methionine-low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst. 1995; 87:265–273. PMID: 7707417.

77. Glynn SA, Albanes D, Pietinen P, Brown CC, Rautalahti M, Tangrea JA, Gunter EW, Barrett MJ, Virtamo J, Taylor PR. Colorectal cancer and folate status: A nested case-control study among male smokers. Cancer Epidemiol Biomarkers Prev. 1996; 5:487–494. PMID: 8827351.
78. Kato I, Dnistrian AM, Schwartz M, Toniolo P, Koenig K, Shore RE, Akhmedkhanov A, Zeleniuch-Jacquotte A, Riboli E. Serum folate, homocysteine and colorectal cancer risk in women: a nested case-control study. Br J Cancer. 1999; 79:1917–1922. PMID: 10206314.

79. Su LJ, Arab L. Nutritional status of folate and colon cancer risk: evidence from NHANES I epidemiologic follow-up study. Ann Epidemiol. 2001; 11:65–72. PMID: 11164122.
80. Martinez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Nurses' Health Study Research Group. Leisure-time physical activity, body size, and colon cancer in women. J Natl Cancer Inst. 1997; 89:948–955. PMID: 9214674.

81. Tavani A, Braga C, La Vecchia C, Conti E, Filiberti R, Montella M, Amadori D, Russo A, Franceschi S. Physical activity and risk of cancers of the colon and rectum: an Italian case-control study. Br J Cancer. 1999; 79:1912–1916. PMID: 10206313.

82. Slattery ML, Edwards S, Curtin K, Ma K, Edwards R, Holubkov R, Schaffer D. Physical activity and colorectal cancer. Am J Epidemiol. 2003; 158:214–224. PMID: 12882943.
83. Sharpe CR, Siemiatycki J, Rachet B. Effects of alcohol consumption on the risk of colorectal cancer among men by anatomical subsite (Canada). Cancer Causes Control. 2002; 13:483–491. PMID: 12146853.
84. Ji BT, Dai Q, Gao YT, Hsing AW, McLaughlin JK, Fraumeni JF Jr, Chow WH. Cigarette and alcohol consumption and the risk of colorectal cancer in Shanghai, China. Eur J Cancer Prev. 2002; 11:237–244. PMID: 12131657.

85. Otani T, Iwasaki M, Yamamoto S, Sobue T, Hanaoka T, Inoue M, Tsugane S. Alcohol consumption, smoking, and subsequent risk of colorectal cancer in middle-aged and elderly Japanese men and women: Japan Public Health Center-based prospective study. Cancer Epidemiol Biomarkers Prev. 2003; 12:1492–1500. PMID: 14693743.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download