INTRODUCTION
Among many factors deciding the prognosis of various cancers, studies reported that tumor size and lymph node status are the most important independent factors of prognosis. Researches have been active on finding the adjunctive factors affecting on the prognosis as a method of finding high recurrent groups such as DNA ploidy, proliferation index, various receptors, oncogenes such as Her-2/neu and p53 and tumor suppressor genes (
1). Especially, studies have been active in the recent years on cell adhesion molecules known to play an important role as the prognostic factors of invasion and metastasis in solid tumors (
2). A very complex process is involved in the mechanism of tumor cell invasion and metastasis. During this process, cell adhesion molecules play the central role in the differentiation of epithelial cells and cytoskeleton maintenance of parenchymal cells. The adhesion between normal cells is achieved through the adherens junction composed of E-cadherin and catenin (
3).
Appropriate binding between cells by adhesion molecules in the differentiation to epithelial cells is very important (
4) and dysfunction in cell adhesion participates in the progression of carcinoma
in situ to infiltrating carcinoma (
5). Dysfunction occurs in adhesion and when changes occur in the expressions or structures of cadherin and catenin, making cells to function as non-adhesive cells or invasive cells. When the expression of E-cadherin is lost, the degree of tumor differentiation is decreased and the possibility of distant metastasis increases, suggesting the role of E-cadherin is inhibiting tumor invasion or metastasis (
6). Catenin is essential in the functions of E-cadherin, brings about strongly invasive characteristic when the expression or structure of E-cadherin fails.
Some studies reported that cyclin D1 is a target of β-catenin in colon cancer (
7) or breast cancer (
8), and the overexpression of cyclin D1 is brought about by activated β-catenin, eventually related with poor prognosis (
9). Furthermore, researchers believe that cyclin D1 is related with ectopic expression of β-catenin such as the expression seen within the nucleus or cytoplasm.
In the present study, we compared 146 cases of head and neck squamous cell carcinoma (HNSCC) with clinical and histologic data such as the degree of histologic differentiation, clinical stage and lymph node status by comparing the degree and pattern of E-cadherin/β-catenin complex expression, and degree of cyclin D1 expression in order to determine the effects of E-cadherin, β-catenin and cyclin D1 on tumor progression and patient prognosis through statistical analysis to aid in predicting patient prognosis and establishing treatment plan.
Go to :

MATERIALS AND METHODS
1) Patients
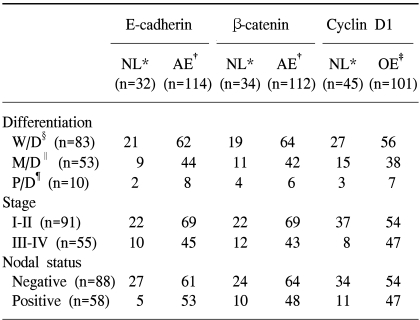
The authors studied 146 consecutive patients with HNSCC who underwent surgical treatment of the primary tumor at the Department of Otorhinolaryngology, Chosun University, from 1994 through 2002. None of the patients had previously received preoperative chemotherapy or radiotherapy. Fifty-eight of 146 patients (39.7%) had histologically confirmed lymph node metastasis, whereas the remaining 88 patients (60.3%) had no clinical or histopathologic evidence of neck disease. Tumors were staged according to the AJCC TNM classification, and graded as follows: well differentiated, moderately differentiated, and poorly differentiated.
2) Immunohistochemical staining
All tumors investigated in the study were tested for mouse monoclonal antibody E-cadherin (clone 36, BD Transduction laboratories, Lexington, KY; dilution 1:200), mouse polyclonal β-catenin (clone-14, BD Transduction laboratories; dilution 1:200) and rabbit polyclonal antibody cyclin D1 (sc-753, Santa Cruz Biotechnology, Santa Cruz, CA; dilution 1:100) using primary tumor. Immunolocalization was performed using a streptavidin-biotin immunoperoxidase method, according to the supplier's protocol (LSAB kit, DAKO, Carpinteria, CA). Briefly, 4µm thick sections were obtained after formalin fixation and paraffin embedding and were deparaffinized in xylene and rehydrated with distilled water through graded concentrations of ethanol. Then the sections were placed in a glass jar with 10 mM citrate buffer (pH 6.0) and irradiated in a microwave oven for 15 minutes, and cooled down in the jar at room temperature for 20 minutes. Then, the slides were rinsed with Tris buffered saline (TBS). After quenching the endogenous peroxidase activity in 0.3% hydrogen peroxide for 10 minutes, blocking reagent was added for 10 minutes. The slides were then washed as before, and were subsequently subjected to the primary antibody reaction. Each primary antibodies was applied in a moist chamber overnight at 4℃. After washing with TBS, a biotinylated link antibody was applied for 10 minutes, followed by streptavidin peroxidase for an additional 10 minutes. After washing out the excess complex, the localization of antibodies was visualized by incubating the sections for 15 minutes in an 3-amino-9-ethyl carbazole (AEC) kit, SK-4200 (Vector laboratories, Burlingame, CA) and counterstaining with Meyer's hematoxylin. An isotype matched control antibody was also used. Positive control for E-cadherin and β-catenin was normal oral mucosa, and that for cyclin D1 was breast carcinoma tissue with strong nuclear staining in another study. Instead of the primary antibody, TBS was used in negative control.
3) Double immunofluorescein staining
Double immunofluorescein staining was done using the same method described previously except after reacting the blocking reagent for 10 min at room temperature without the treatment with hydrogen peroxide, the sample was tapped off of excess blocking reagent and reacted with the primary antibody. Double staining of E-cadherin and β-catenin was done with mixtures of mouse monoclonal antibody E-cadherin (clone 36, BD Transduction laboratories, dilution 1:100) and rabbit polyclonal antibody β-catenin (Ab-1, NeoMarkers, Fremont, CA, dilution 1:100) at 4℃ for 24 h. Double staining of β-catenin and cyclin D1 was done with mixture of mouse polyclonal antibody β-catenin (clone-14, BD Transduction laboratories; dilution 1:100) and rabbit polyclonal antibody cyclin D1 (sc-753, Santa Cruz Biotechnology; dilution 1:100) at 4℃ for 24 h. After washing several times with Tris buffer, the immune marker, secondary antibody mixture of fluorescein anti-mouse Ig G (H+L) (FI-2000, Vector laboratories; dilution 1:100) and Texas-Red anti-rabbit Ig G (H+L) (TI-1000, Vector laboratories, dilution 1:100) was reacted at 4℃ for 3 h in dark and washed for 3 h in dark using Tris buffer. The slide was then wet mounted. The negative control was tissue reacted only with the secondary antibody while omitting the primary antibody. The stained slides were observed immediately under a confocal laser scanning microscope (FV300, Olympus, Japan) and images were obtained.
4) Analysis and interpretation of staining
E-cadherin and β-catenin immunoreactivities were evaluated separately for the cell membrane, cytoplasmic and nuclear compartments. The membranous expression of E-cadherin or β-catenin in cancer tissue was defined as 'normal' or 'preserved' expression if more than 70% of tumor cells was stained by the antibody. If less than 70% of tumor cells were stained, the membranous expression was defined as 'reduced'. On the other hand, positive cytoplasmic or nuclear immunoreactivity of E-cadherin or β-catenin was judged if more than 10% of tumor cells were positive. 'Reduced', cytoplasmic or nuclear immunoreactivity was recategorized into 'aberrant' expression.
Staining for cyclin D1 was determined positive when nuclear protein was stained red brown under optical microscope, was negative when nuclear staining was present less than 5% in the area of tumor cells, overexpression when more than 5% of tumor cell nuclei was stained positive. The immunoreactivities were evaluated in three randomly selected high-power fields in the periphery of the tumor. Necrotic areas were not taken into consideration. All tumor slides were examined by two investigators who were unaware of the clinical data.
All of double immunofluorescein stained tissue samples were observed under a confocal laser scanning microscope. As for the excitation wave length of laser, 488 nm was used for FITC and 568 nm for Texas-red. Scan images were obtained at an interval between 0.5µm or 1µm, and final 3-D images were reconstructed using a flow view software program (Olympus), stored in computer and printed for use. Strong double staining was determined when bright yellow color was seen.
5) Statistical analysis
SPSS (statistical package for the social sciences), Windows, version 7.5 (SPSS, Korea) was used for statistical analysis. X2 test was used to determine the correlation between clinical stage, degree of histologic differentiation, and lymph node metastasis and expression patterns of E-cadherin, β-catenin, and cyclin D1; correlation in the expression patterns among E-cadherin, β-catenin, and cyclin D1. Statistical significance was determined at P<0.05.
Go to :

DISCUSSION
Various clinicopathologic data provided when diagnosing various cancers have been used as important data in establishing the direction of treatment and predicting patient prognosis. However, these data are not always reliable so that researches have been focused on finding more objective and reliable diagnostic and prognostic markers (
10). When we could differentiate low risk- and high risk-patients according to the ideal marker, treatment outcomes could be increased drastically in high risk-patients through more aggressive treatment such as surgery combined with adjunctive radiotherapy, chemotherapy or hormone therapy and various economic and medical damages could be reduced by preventing unnecessary treatment in low risk patients (
11). Among the researches on finding reliable adjunctive prognostic markers, researches are active on adhesion molecules in solid tumors that are known to play an important role as prognostic factors related with tumor invasion and metastasis (
2,
12,
13).
Cadherin is divided into types N, P, and E according to immunologic characteristics and histologic distribution and E-cadherin is the most important mediator in maintaining the epithelial structure forming the cadherin/catenin complex within cells by binding to catenin. This function of E-cadherin was proven experimentally in which intercellular binding increases so that tumor invasiveness could be prevented when E-cadherin cDNA is transplanted in cell lines with poor prognosis having no expression of E-cadherin and tumor invasiveness could be recovered with the introduction of E-cadherin antibody (
14). From the aspect of E-cadherin function, tumor growth shows the pattern of expansile growth, which is the growth pattern of benign tumors, without showing the growth pattern of invasive tumor even in the presence of malignant tumors when intercellular binding is maintained through normal action of E-cadherin (
4,
5). Therefore, malignant transformation followed by the change in intercellular binding is essential in invasion and expansile growth, and the lack of various cell boundary structures participating in the intercellular adhesion is observed in various cancers (
15).
Catenin present as α-catenin (102 kDa), β-catenin (88 kDa), and γ-catenin (80 kDa) is an anchoring protein present in cytoplasm and is essential in maintaining normal functions of E-cadherin in the cross-linkage action between actin filament and the intracellular membranous proteins, Na
+/K
+ adenosine triphosphatase and E-cadherin (
3). Among these molecules, α-catenin having the similar base sequence as vinculin plays the role of binding actin filament to cadherin bonded with β- or γ-catenin (
5). However, β-catenin binds directly to the cytoplasmic domain of E-cadherin molecule similar to γ-catenin. This binding is essential in stable cell-cell adhesion and is partially controlled by β-catenin. At the same time, β-catenin plays the role of an important marker in the signal transduction mediated by the protooncogene byproduct c-erbB2 and epidermal growth factor receptor (EGFR) (
16). On the other hand, the functions of β-catenin would differ according to its location within cells. Cytoplasmic β-catenin, other than the previously mentioned membrane-bound form, is soluble, and is responsible for the Wnt/wingless signaling pathway, and is inhibited by normal adenomatous polyposis coli (APC) protein and glycogen synthetase kinase (GSK)-3-beta (
17). When β-catenin is translocated outside of the nucleus, it forms a complex with T-cell specific transcription factor (TCF)/lymphoid-enhancer-binding factor (LEF) transcription factor. Thus, nuclear β-catenin related with TCF/LEF activates the transcription of various target genes including c-myc, c-jun, fra-1, urokinase-type plasminogen activator receptor and cyclin D1, brought about by the mutation of β-catenin, deletion of APC gene, and activation of Wnt pathway (
7,
18). In other words, some showing the overexpression of cyclin D1 is not related with the gene amplification of cyclin D1. In these cases, it was believed that most are brought about by the strengthening of transcriptional activity of the cyclin D1 gene (
12). However, cyclin D1 is overexpressed in all cases showing cyclin D1 gene amplification (
9).
The adhesion molecules E-cadherin and β-catenin were stained uniformly in most normal epithelial cells around the tumor, showing normal location of adhesion molecules in the cell membrane. However, changes of E-cadherin and β-catenin expressions were seen in 79% and 78%, respectively, in HNSCC. Eventually, it was presumed that the aberrant expression occurs in adhesion proteins within normal cell membrane in many cases of HNSCC so that adhesion molecules become stronger or weaken to bring about the ectopic expression in the cytoplasm or nucleus. According to the previous studies, decreased expressions of E-cadherin and β-catenin in esophagus or bladder carcinoma are significantly related with histologic differentiation and metastasis (
19). It was reported that the expressions of E-cadherin and β-catenin were negative in colonic adenocarcinoma invading the colon wall but the cases maintaining the tumor glands were frequently seen (
20). Thus, the fact that E-cadherin and β-catenin affect differently according to organs or individuals suggests that other cadherin proteins such as OB-, P-, and R-cadherin expressed in epithelial cells at the applicable site are compensating for functions, that adhesion among cells becomes weak despite intercellular binding of tumor cells, and that the binding structure formed is not suffice so that these different effects are seen according to the degree of variables affecting (
21).
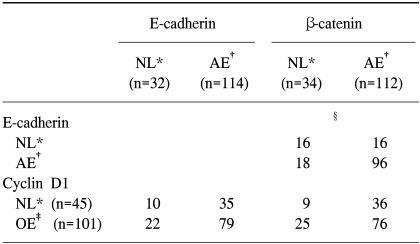
As for the relationship between E-cadherin and β-catenin in the present study, the expression of β-catenin was also aberrant when aberrant expression of E-cadherin was present, and the expression of β-catenin was also maintained when the expression of E-cadherin was normal. Furthermore, when E-cadherin was compared according to the pattern of expression, significant difference was present when the expression of E-cadherin was normal or abnormal in the absence or presence of lymph node metastasis, respectively. However, there was no correlation between expression pattern of β-catenin and differentiation, clinical stage or nodal status. Nevertheless, the pattern of β-catenin expression showed a very close relationship with the expression pattern of E-cadherin. This finding suggests some relationship between the direct down-regulation of the cadherin/catenin complex and metastasis of HNSCC. The E-cadherin and β-catenin proteins are directly connected so that abnormality in these proteins suggests the adhesion of tumor cells. Nonetheless, the exact mechanism has not been determined. When even one component among the components of the E-cadherin/β-catenin complex is not functioning properly, the functions of the complex are greatly affected, affecting various clinicopathological indices (
20). This result agrees with the finding that the loss of cell adhesion occurred when a mutation occurs in the β-catenin gene even when the expression of E-cadherin was normal in stomach cancer cell lines (
22).
Some studies reported that the ectopic expression of β-catenin affects the expression of cyclin D1 (
12,
18). No significant correlation was found between these two expressions according to the findings of the present study. Furthermore, some studies reported that the prognosis is poor when the expression of β-catenin is present within the nucleus in squamous cell carcinoma of the pharynx and hepatoblastoma, and a study reported that the expression of β-catenin in the cytoplasm is a predictor of hematogenous metastasis in colorectal carcinoma (
2). Thus, the possibility of β-catenin overexpression or ectopic expression was suggested during this process since cellular adhesion is needed for tumor cells freed from blood vessels in the primary lesion to settle in a new site. In the present study, no significant correlation was present between changes in β-catenin expression and lymph node metastasis. Moreover, no significant correlation was present between the presence of ectopic expressions of β-catenin in the cytoplasm and nucleus, degree of histologic differentiation, clinical stage, and degree of lymph node metastasis. However, β-catenin separate from the action by E-cadherin interacts with EGFR to participate in the phosphorylation of tyrosine; thus, it could show somewhat different pattern from E-cadherin.
On the other hand, a strong membranous expression of E-cadherin and/or β-catenin was observed according to immunohistochemistry in some cancer cells compared with normal cells. In this case, abnormality occurred in the adhesion site although the expression increased according to staining so that it was possible that the binding power decreased functionally. Also in the present study, the membranous expression was increased in those subjects showing clinically advanced cancer (stage III/IV) at no significance. Although this increase was possibly increased due to increased reactivity, we could not exclude the possibility of the amplification by gene mutation or activation by transcription.
Among different cyclins, cyclin D1 reported to be most related with tumor formation is a protein essential in the switch from G1 to S stage during the cell cycle and plays the role of detecting cellular proliferating signal. It is overexpressed in various malignant tumors and is suggested as a useful index of prognosis. It was reported that cyclin D1 is strictly regulated in normal cells but increases and forms a complex with cdk4 to bring about cellular proliferation and deformity when external stimulation is present, especially, when growth factors are stimulated (
23).
Overexpression of cyclin D1 was reported to differ according to organs and researchers. The studies reported that the overexpression of cyclin D1 is related with poor prognosis in squamous cell carcinoma of the larynx and hypopharynx (
24), is related with good prognosis in breast cancer, is not related with good prognosis in breast cancer, and does not have a prognostic value in non-small cell lung cancer (
25). The amplification of cyclin D1 gene is seen in about 10~20% in breast cancer cases; however, the overexpression of cyclin D1 protein varies from 35~80% according to different authors. Thus, the fact that the overexpression of cyclin D1 protein is higher compared with the gene amplification suggests that the expression of cyclin D1 protein could be brought about the mechanism other than the gene amplification, which is resulted from mutation or translocation and could result from the increased sensitivity to estrogen in breast cancer (
25). The overexpression of cyclin D1 was seen in 69% according to the present study, but no correlation was seen in the expressions of cyclin D1 and E-cadherin, and cyclin D1 and β-catenin. Furthermore, when correlation between cyclin D1 and various clinicopathologic indices was examined, a significant correlation was seen in clinical stage and lymph nodal status but in histologic differentiation.
In summary, the degree of histologic differentiation, clinical stage, lymph node metastasis were observed in respect to the expressions of E-cadherin/β-catenin complex and the degree and pattern of cyclin D1 in HNSCC, were analyzed through immunohistochemstry, confirmed under a confocal scanning laser microscope, and analyzed statistically to obtain the following results. The rate of aberrant expression was 79% for E-cadherin and 78% for β-catenin, and the rate of overexpression of cyclin D1 was 69%. The aberrant expression of E-cadherin was significantly correlated with the lymph nodal status. A significant correlation was present between the overexpression of cyclin D1 and the lymph nodal status or clinical stage. A significant correlation was seen in normal and aberrant expressions between E-cadherin and β-catenin in which an aberrant expression of one molecule was seen with aberrant expression of the other molecule. However, no statistically significant relationship was present in overexpression and aberrant expression between cyclin D1 and E-cadherin or between cyclin D1 and β-catenin.
Go to :






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download