Abstract
Purpose
The purpose of this study was to evaluate the efficacy of intrapleural chemotherapy (IPC) with cisplatin and cytarabine in the management of malignant pleural effusion (MPE) from non-small-cell lung cancer (NSCLC).
Materials and Methods
A prospective analysis was carried out on 40 patients with pathologically proven MPE from NSCLC who had received IPC. A single dose of cisplatin 100 mg/m2 plus cytarabine 1200 mg/m2 in 250 ml normal saline was instilled into the pleural space via a chest tube and drained 4 hours later. Patients were evaluated for toxicities and responses at 1, 2, & 3 weeks and then at monthly intervals if possible. Systemic chemotherapy was administered, if the patient agreed to receive it, after achieving complete control (CC) of MPE.
Results
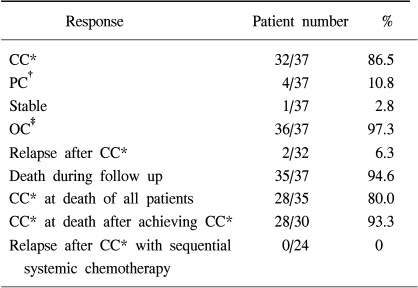
The median duration of chest tube insertion for drainage was 7 (3~32) days. Among the assessable 37 patients, CC and partial control (PC) were 32 (86.5%) and 4 (10.8%) patients, respectively (overall response rate 97.3%). The median duration of response was 12 months (2~23) and there were only two relapses of IPC after achieving CC. Among the 35 patients who were assessable until they died, 28 patients (80.0%) maintained CC until the last follow-up. There was only one toxic death and the toxicities of IPC, versus the results obtained, were deemed acceptable.
Go to : 
Malignant pleural effusion (MPE) usually causes significant problems in patients with advanced malignancies (1~3). When the underlying cancer is likely to be highly sensitive to systemic chemotherapy, such a therapy might be the treatment of choice. However, when the underlying cancer is unlikely to be responsive to systemic chemotherapy, as in NSCLC, tube thoracostomy with subsequent pleurodesis has usually been recommended. Unfortunately, more often than not, the tumor causing the malignant effusion is not sensitive to systemic chemotherapy and is either partially sensitive or progressive by nature. Control of MPE may greatly improve the quality of life of the patient under such circumstances. In contrast to traditional sclerosing agents (4), IPC has the potential advantage of treating the underlying malignancy in addition to providing local control of MPE (5).
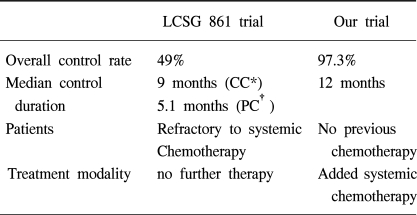
In 1994, the Lung Cancer Study Group (LCSG) (6) evaluated and reported the effectiveness of intrapleural cisplatin and cytarabine in patients with MPE caused by a variety of solid malignancies. The overall response rate was only 49% in patients with MPE who were refractory to standard systemic therapy. Other investigators reported relatively good response results to IPC in patients with different eligibility criteria. IPC with cisplatin and cytarabine, mitoxantrone (7), or paclitaxel (8) were reported to induce an overall response rate of 74~93% with acceptable toxicity.
This study was carried out to evaluate the efficacy of IPC using cisplatin and cytarabine for the management of MPE caused by NSCLC.
Go to : 
Patients were eligible for this study if they had pathologically proven MPE associated with NSCLC. Patients selected were 18 years of age or older. A life expectancy of 2 months or longer and a performance status of 0~2 on the ECOG scale were required. No previous intrapleural therapy was a requirement, as was no systemic chemotherapy, radiotherapy and hormonal therapy within the 4 weeks prior to trial entry. Adequate hepatic, renal, and hematologic functions were also required for entry into this study. The parameters used for these functions were total bilirubin <1.5×(the upper normal value) (N), creatinine clearance≥60 ml/min, peripheral leucocyte count> 4,000/µl, and a platelet count> 140,000/µl. Informed consent was obtained from all patients before entry to the study.
Patients were admitted to the hospital and given pretreatment hydration with 2,000 mL of normal saline and furosemide. All patients received premedication for cisplatin chemotherapy, including an intravenous infusion of granisetron (3 mg) and dexamethasone (10 mg), 30 min prior to IPC. 250 mL of normal saline containing cisplatin 100 mg/m2 and cytarabine 1,200 mg/m2 was then instilled into the pleural space via a chest tube and drained 4 hours later. The patient changed position every 15 to 20 minutes during that time, in order to assure good dispersion of the drugs throughout the pleural space. A drainage rate of less than 100 ml/24 hours was required for the removal of the chest tube.
A baseline chest X-ray and decubitus radiograph or chest CT were obtained. All patients were evaluated for toxicity and response with follow up decubitus radiographs or chest CTs at 48hrs, the 1st, 2nd, and 3rd weeks and then monthly intervals, where possible, after removal of the chest tube. Toxicity was graded according to the WHO Toxicity Criteria. Total disappearance of pleural fluid, or residual pleural fluid too minimal to be approachable by thoracentesis, was considered as complete control (CC). Partial control (PC) was defined as a 75% or greater reduction of pleural effusion while stabilization, as less than a 75% reduction with no increase in the amount of pleural fluid, as compared to baseline pretreatment chest radiography. Progression was defined as a greater than 25% increase of pleural fluid as compared to baseline chest radiography. An objective response of MPE was either CC or PC. Systemic chemotherapy could be initiated 3 weeks after IPC, according to the disease status in those patients with an objective response.
Go to : 
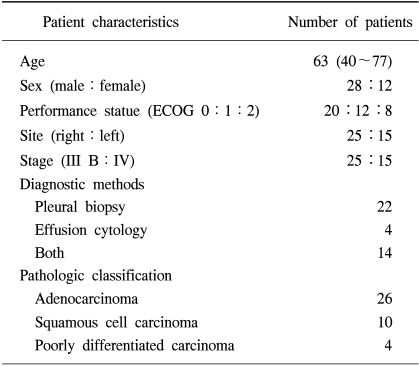
From Jan 1997 to Dec 2002, a prospective analysis of 40 patients with symptomatic, pathologically proven MPE from NSCLC was carried out to estimate the effect of IPC. Table 1 shows the clinical characteristics of these patients. There were 28 males and 12 females with a median age of 63 years (40~77). The pathologic classification of NSCLC included 26 adenocarcinomas, 10 squamous cell carcinomas and 4 poorly differentiated carcinomas. All patients had no previous systemic chemotherapy or local radiotherapy.
A total of 37 of the 40 patients were assessable for follow-up investigation (Table 2). The median follow up period was 6.5 (2~23) months. Among the assessable 37 patients, CC and PC were found in 32 (86.5%) and 4 (10.8%) patients, respectively and the overall objective response rate was 97.3%. All patients received just one cycle of IPC. Treatment failed one patient only, salvage sclerotherapy with doxycycline was needed to control the MPE because of poor tolerance to chemotherapy. The median duration of response to IPC was 12 months (2~23) and there were only two relapses of IPC treated patients after achieving CC. Among the assessable 37 patients, a total of 35 patients died during the study. In most of the patients, the causes of death were related to progression of a primary lung mass. Among 35 patients who were assessable until their demise, 28 patients (80.0%) maintained CC at the last follow-up. All 4 patients who had experienced PR after IPC relapsed until they died.
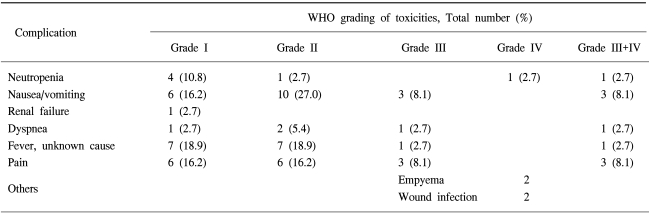
In the 24 patients with NSCLC who had received sequential systemic chemotherapy after achieving CC of MPE, there was no experience of relapse into MPE until they either died or until the last follow up examination. Eight patients refused further systemic chemotherapy and 6 of these patients had no relapse until they either died or until the last follow up examination. Table 3 illustrates the major toxicities. Three patients died within 4 weeks of IPC treament but there was only one treatment-related death (2.5%). The cause of death in this patient, was aggravation of heart failure after IPC. The deaths of the other two patients did not seem to be related to IPC treament. The cause of death in these patients was a massive cerebral infarction and a traffic accident, respectively. One patient experienced reversible grade 4 myelosuppression, 3 patients had grade 3 nausea and vomiting. Two patients had empyema and 2 patients had wound infection. There was no significant renal and hepatic toxicity. Pain was relatively tolerable and managed with NSAID or narcotic therapy.
Go to : 
MPE is a common problem in patients with malignancy and may be one of the major causes of treatment failure in conditions with underlying malignancies. It is well known that systemic chemotherapy and radiotherapy may not help to control MPE. Among the many different sclerosing agents for MPE, tetracycline (9,10) has been widely used. Tetracycline has disadvantages, such as, causing severe pain, a low frequency of inducing CC in a substantial number of patients and ineffectiveness in treating the underlying malignancy. Other sclerosing agents used for chemical pleurodesis are talc (11), doxycycline (12), Corynebacterium parvum (13), bleomycin (14), and OK-432 (15). The success rates of these agents are reported to be 50~80%, except for talc. The success rate of talc pleurodesis is over 90% but this is an invasive procedure which includes thoracoscopy and general anesthesia.
There are a few reports concerning IPC. Chemotherapeutic agents that have been used in the past include, nitrogen mustard, doxorubicin, methotrexate, mitoxantrone, 5-fluorouracil, cisplatin, mitomycin, cytarabine, etoposide and paclitaxel, etc. The LCSG 861 study concluded that IPC with cisplatin and cytarabine is not recommended for standard treatment of MPE due to its low response rate (49%) but could potentially play a role in multimodality cancer treatment. The outcome of this present trial indicates that IPC with cisplatin and cytarabine has a high response rate (97.3%) and long term treatment response (median duration of response 12 months). The procedures and chemotherapy-induced complications were within tolerable limits with little treatment-related mortality and morbidity. The discordant results between the LCSG 861 study and this study seem to be caused by different eligibility criteria and treatment strategies between the studies (Table 4). The LCSG 861 study included patients with MPE who were refractory to systemic chemotherapy and did not receive further sequential treatments after IPC. The present study applied IPC as an initial treatment to those patients with MPE from NSCLC before systemic chemotherapy and recommended further sequential systemic chemotherapy according to disease status and performance. Other investigators reported the relatively good response results of IPC. The eligible criteria of those studies were very similar to this study. They reported that IPC with cisplatin and cytarabine (16,17), mitoxantrone (7) or paclitaxel (8) induced an overall response rate of 74~93% with an acceptable toxicity level. In the present study, 24 patients with NSCLC received sequential systemic chemotherapy after CC of MPE was achieved with IPC and no patients experienced a relapse of MPE until their death. Among 8 patients who refused further systemic chemotherapy, 6 patients experienced no relapse until their death. However, the difference in the relapse rate between two groups treated by IPC, with or without sequential systemic chemotherapy, was not able to be discerned.
There did not appear to be any potential advantage in treating the underlying malignancy, including systemic antitumor treatments. Most of the patients who had received IPC had no pleural effusion until their death. These results suggest that IPC could be incorporated into multimodality cancer treatment in patients with MPE, in order to reduce effusion related symptoms and to increase the response when treated with systemic treatment.
As a result, it is suggested that IPC with cisplatin and cytarabine may well be considered as the standard treatment in those patients with MPE, not only as a palliative but also as one component of a multimodality cancer treatment against underlying malignancies.
Go to : 
This study attempted to evaluate the efficacy of IPC with cisplatin and cytarabine in the management of MPE from NSCLC. The procedures were tolerable and any chemotherapy-induced complications were acceptable. The outcome of this trial indicates that IPC has a worthwhile and long term treatment response in the management of patients with MPE from NSCLC.
Go to : 
References
1. Hausheer FH, Yarbro JW. Diagnosis and treatment of malignant pleural effusion. Semin Oncol. 1985; 12:54–75. PMID: 2579439.

2. Ruckdeschel JC. Management of malignant pleural effusion: An overview. Semin Oncol. 1988; 15(suppl 3):24–28. PMID: 3293215.
3. Moores DWO. Malignant pleural effusion. Semin Oncol. 1991; 18(suppl 2):59–61. PMID: 1704153.
4. Walker-Renard PB, Vaughan LM, Sahn SA. Chemical pleurodesis for malignant pleural effusions. Ann Intern Med. 1994; 120:56–64. PMID: 8250457.

5. Rusch VW, Figlin R, Godwin D, Piantadosi S. Intrapleural cisplatin and cytarabine in the management of malignant pleural effusions: A lung cancer study group trial. J Clin Oncol. 1991; 9:313–319. PMID: 1988578.

6. Figlin R, Mendoza E, Piantadosi S, Rusch V. Intrapleural chemotherapy without pleurodesis for malignant pleural effusions: LCSG Trial 861. Chest. 1994; 106(suppl):363s–366s. PMID: 7988265.

7. Aasebo U, Norum J, Sager G, Slordal L. Intrapleurally instilled mitoxantrone in metastatic pleural effusions: A phase II study. J Chemother. 1997; 9:106–111. PMID: 9176748.
8. Perng RP, Chen YM, Wu MF, Chou KC, Lin WC, Liu JM, et al. Phase II trial of intrapleural paclitaxel injection for non-small-cell lung cancer patients with malignant pleural effusions. Respir Med. 1988; 92:473–479. PMID: 9692108.

9. Rubinson Rm, Bolooki H. Intrapleural tetracycline for control of malignant pleural effusion: A preliminary report. South Med J. 1972; 65:847–849. PMID: 5038178.
10. Gravelyn TR, Michelson MK, Gross BH, Sitrin RG. Tetracycline pleurodesis for malignant pleural effusions: A ten-year retrospective study. Cancer. 1987; 59:1973–1977. PMID: 2436744.
11. Hartman DI, Gaither JM, Kesler KA, Mylet DM, Brown JW, Mathus PN. Comparison of insufflated talc under thoracoscopic guidance with standard tetracycline and bleomycin pleurodesis for control of malignant pleural effusions. J Thorac Cardiovasc Surg. 1993; 105:743–748. PMID: 7682268.

12. Mansson T. Treatment of malignant pleural effusion with doxycycline. Scand J Infect Dis Suppl. 1988; 53:29–34. PMID: 3166542.
13. Hillerdal G, Kiviloog J, Nou E, Steinholtz L. Corynebacterium parvum in malignant pleural effusion: A randomized prospective study. Eur J Respir Dis. 1986; 69:204–206. PMID: 2430825.
14. Paladine W, Cunningham TJ, Sponzo R, Donavan M, Olson K, Hoton J. Intacavitay bleomycin in the management of malignant effusions. Cancer. 1976; 38:1903–1908. PMID: 62609.
15. Lim HY, Kim JH, Park YH, Chung HC, Choi JJ, CHoi SG, Kim HG, Choi JH, Yoo NC, Koh EH, Chang J, Roh JK, Kim SK, Lee WY, Youn JK, Kim BS, Kim JJ. Intrapleural instillation of OK-432 for malignant pleural effusion. J Korean Cancer Assoc. 1992; 24:47–55.
16. Markman M, Cleary S, King ME, Howell SB. Cisplatin and cytarabine administered intrapleurally as treatment of malignant pleural effusions. Med Pediatr Oncol. 1985; 13:191–193. PMID: 4040205.

17. Aitini E, Cavazzini G, Pasquini E, et al. Treatment of primary of metastatic pleural effusion with intracavitary cytosine arabinoside and cisplatin. A phase II study. Acta Oncol. 1994; 33:191–194. PMID: 8204275.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download