Abstract
Purpose
E-cadherin, a calcium-dependent cell to cell adhesion molecule, plays a key role in the maintenance of tissue integrity. Reduction or loss of E-cadherin has been reported to have a role in the development of human malignancies. The expression of E-cadherin was analyzed in human non-small cell lung carcinoma (NSCLC) to elucidate the role in pulmonary carcinogenesis and determine the relationship with several clinicopathological factors and the prognosis.
Materials and Methods
Sixty five human cases of NSCLC were evaluated by immunohistochemical analysis for the expression of E-cadherin. The immunostaining results for E-cadherin were semiquantitatively interpreted, as preserved and reduced, in the tumor tissues. The E-cadherin expression was analyzed in relation to several clinicopathological data and the survival. The cell proliferation index of the tumors was evaluated by immunostaining with the Ki-67 antigen.
Results
Reduced E-cadherin expression was found in 51 cases of NSCLC tissues (78.4%) compared to that in the normal controls. Reduced E-cadherin expression was significantly correlated with male smokers and squamous cell type of the cancer, but not with histological grade, TNM stage and survival. The E-cadherin expression showed a weak inverse relationship with the proliferative activity of tumor cells, which was measured using the Ki-67 antigen.
Go to : 
The loss of cell-cell adhesion and invasion of carcinoma cells into the surrounding mesenchymal tissue have been associated with the malignant phenotype for more than 50 years (1). One important area of research has been the characterization of cell-cell and cell-substratum adhesion molecules. E-cadherins are a class of calcium-dependent transmembrane cell adhesion molecules (CAM) that mediate cell-cell interactions via homophilic interactions (2). E-cadherin is expressed on the cell surface in most epithelial tissues, and is important in the maintenance of epithelial integrity and cellular differentiation. It has also been implicated in carcinogenesis because its expression is frequently lost in human epithelial cancers (3).
Recently, many studies have shown that E-cadherin is reduced in various human tumors, such as esophagus, stomach, colon, liver, pancreas and urinary bladder, and is related to tumor progression, metastasis and prognosis (4~9). In non-small cell lung carcinomas (NSCLC), several studies have suggested that reduction and/or loss of E-cadherin expression is responsible for the development of a malignant phenotype (1,10). Moreover, recent clinical studies have shown that reduced E-cadherin is associated with tumor dedifferentiation (11~15) and lymph node metastasis (12~16) as well as unfavorable prognosis in patients with NSCLC (11,12,14,15).
To find out whether E-cadherin expression is involved in the pathogenesis of NSCLC and associated with any significant clinicopathological parameters, immunohistochemical analysis of E-cadherin in the 65 resection specimens of NSCLC and corresponding paracarcinoma controls were performed.
Go to : 
65 tumor specimens were obtained from patients undergoing pulmonary resections for NSCLC, between 1996 and 2001, at the Dankook University Hospital. All specimens used in this study were 4µm-thick sections of paraffin-embedded tissue obtained at the resection of NSCLC. Formal pathology reports were obtained for each specimen to document the tumor cell types, according to the WHO diagnostic criteria for lung carcinomas (1999) and differentiation (well, moderately well, and poor). The hospital records of all 65 patients were reviewed to obtain the clinicopathological variables, such as age, gender, smoking history and TNM stage. The pathological staging of NSCLC was assessed according to the TNM classification of the AJCC staging system (1997). Death from lung cancer was the terminal event for survival calculations. All patients were followed up for at maximum 76 months.
The standard avidin-biotin-peroxidase complex method was used for immunohistochemical examination, using the monoclonal antibody against E-cadherin (4A2C7, Zymed, CA) and the polyclonal antibody for Ki-67 (A047, DAKO, Carpinteria, CA).
Deparaffinization of all sections was performed through a series of xylene baths, and rehydration was performed through graded alcohols. The sections were microwaved in 10mM citrate buffer at 90℃ for 10 min, and then treated with 3% H2O2-PBS solution to reduce the endogenous peroxidase activity. They were then incubated with normal bovine serum to reduce nonspecific antibody binding, and subsequently subjected to the primary antibody reactions. The antibodies for E-cadherin and Ki-67 proteins were reacted with the sections at room temperature for one hour, at the dilutions of 1:50 and 1:100, respectively. Detection of the immunoreactive staining was carried out by the avidin-biotin-peroxidase complex method, using the LSAB kit (DAKO). The sections were subjected to a color reaction with 3, 3-diaminobenzidine tetrahydrochloride, containing 3% H2O2 in Tris buffer, and lightly counterstained with Mayer's hematoxylin.
For evaluation of the expressions of E-cadherin and Ki-67 proteins, the immunostained cells were considered positive only when distinct membranous and nuclear stainings, respectively, were identified. Normal bronchial epithelium adjacent to the tumor was used as a positive control for the evaluation of E-cadherin expression in the tumor cells.
According to the criteria used by Bohm et al. (17), the level of E-cadherin expression was classified as 'preserved' when more than 90% of the tumor cells were stained, as 'reduced' when less than 90% of the tumor cells were stained and as 'negative' when the tumor cells completely lacked membranous staining. In our study, however, only one case showed 'negative' staining. Thus, the negative case was included in the 'reduced' group.
Cases were considered 'high labeling' for Ki-67 expression when more than 10% of tumor cells were reactive, as the median value of Ki-67 proliferative fraction in all cases was about 10%.
The comparison of reduced expression between several two-categorical clinicopathological variables and the relationship of E-cadherin expression with proliferative activity, as measured by the Ki-67 reactivity, were analysed by chi-squared tests (SPSS 10.1). The survival period was calculated as the time from the date of surgery to the date of death or the last follow-up. Postoperative survival curves were constructed using the Kaplan-Meier method, and then compared by log-rank tests. A p value less than 0.05 was defined as statistically significant.
Go to : 
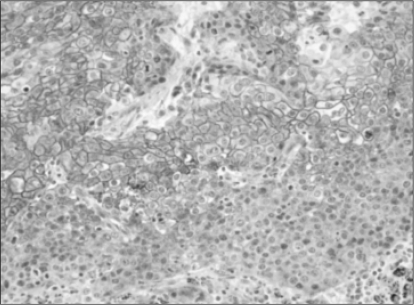
The non-neoplastic lung, alveolar and bronchial epithelial cells showed distinct membranous staining (Fig. 1). No substantial differences were observed between the non-neoplastic and malignant cells in the staining patterns with the antibody to E-cadherin, but the tumor cells generally showed weaker and heterogeneous expression of E-cadherin.
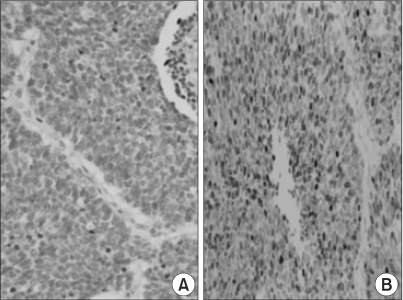
Overall, of the 65 NSCLC cases, 51 (78.4%) showed reduced or negative immunoreactivity for E-cadherin. Reduced E-cadherin expression was found in 28 of the 30 squamous cell carcinomas (93%), 15 of the 25 adenocarcinomas (60%), 3 out of 3 adenosquamous carcinomas (100%), 1 of the 2 bronchioloalveolar carcinomas (50%), 3 out of 3 large cell carcinomas (100%) and 1 of the 2 pleomorphic carcinomas (50%). The squamous cell carcinomas revealed more distinct membranous staining in the central areas of tumor cell nests, where better differentiation was shown than in the peripheral portions (Fig. 2). However, the adenocarcinomas showed no difference in the staining intensities according to the areas with variable differentiation (Fig. 3).
The patient profiles are shown in Table 1. The NSCLC patients consisted of 49 men and 16 women, with median ages of 58.0 and 61.8 years, respectively. Overall, the median age at diagnosis was 59.9, ranging from 31 to 83 years. The pathological staging of the 65 cases revealed 25 cases of stage 1, 10 of stage 2 and 30 of stage 3. The pathologic T stages were pT1 in 14 cases, pT2 in 31, pT3 in 16 and pT4 in 4. The pathologic N stages were pN1 in 41 cases, pN2 in 23 and pN3 in 1. The histological types included 30 squamous cell carcinomas, 25 adenocarcinomas, 3 adenosquamous, 2 bronchioloalveolar carcinomas, 3 large cell carcinomas and 2 pleomorphic carcinomas, based on the WHO classification (1999). The adenocarcinomas and squamous cell carcinomas were graded as 1 (well differentiated) in 6 cases, 2 (moderately well differentiated) in 36 and 3 (poorly differentiated) in 13.
The E-cadherin expression was overall reduced in 51 of the 65 cases (78.4%), and not correlated with most of the clinicopathological variables, including age, TNM stage, nodal metastasis, tumor size and histological differentiation. However, the E-cadherin expression was significantly reduced in males and smokers. Also, the histological type of SCC showed significantly reduced E-cadherin expression than the adenocarcinomas (p=0.007).
The statistical analysis for the relationship is shown in Table 2. There was a marginally significant correlation between E-cadherin expression and the Ki-67 proliferative activity (p=0.066). The reduced group for E-cadherin expression tended to reveal higher proliferative activity than the preserved group (Fig. 4).
Complete follow-up information was available on 36 of the 65 NSCLC cases, with a median follow-up duration of 37.7 months for those living (range, 12~76 months). When all 65 of the NSCLC patients analyzed for postsurgical survival were examined for differences in the survival times between the preserved and reduced E-cadherin expression groups, by the Kaplan-Meier method and log-rank tests, no significant difference in their survival times were found.
Go to : 
Several recent studies have proposed that abnormality in the expression of E-cadherin plays an important role in NSCLC carcinogenesis (1,11). In this study, the immunohistochemical expression for E-cadherin was evaluated in the 65 NSCLC cases and paracarcinoma controls to elucidate the role of E-cadherin in pulmonary carcinogenesis. Our results showed that the E-cadherin expression was reduced or absent in the vast majority of the 65 NSCLC cases (78.4%). Of the 65 carcinomas, 51 (78.4%) showed absent or reduced expression, and 14 (21.6%) showed preserved expression, of E-cadherin proteins. Previous studies reported that the rate of impaired expression in non-small cell lung carcinomas ranged from 42 to 81% (10,12,13,15~19). According to Bohm et al. the E-cadherin expression level was classified as reduced when florescence intensity was markedly less than that of the adjacent normal epithelium and/or 90~95% of the tumor cells were stained, and as absent when the staining was not distinguishable from background or <5% of the tumor cells were stained, with the result showing 53% were the reduced or absent types (19). The same criteria to those of Bohm et al. were used, as malignant tumors generally show less and more heterogeneous expressions of the E-cadherin adhesion complex, compared with normal tissues. When 90% was used as the criterion, 78.4% were shown to be the reduced or absent types, which was higher than other investigations for NSCLC (10,12,13,15~19). However, it is difficult to directly compare the present result with each of the previous studies because of the inherent semi-quantitative nature of immunohistochemical analyses. The specific antibodies used, and their concentrations, techniques in tissue procurement and types of tumors analyzed will affect the results. Most importantly, each group analyzed and interpreted their immunohistochemical data in slightly different fashions. Despite the fact that the results of each of these studies were generally lower than ours, the finding of E-cadherin down-regulation in the vast majority of lung carcinomas was consistent with the hypothesis that this molecule plays an important role in the development of a malignant phenotype.
Previous clinical studies on cancer have revealed that reduced E-cadherin expression is associated with tumor dedifferentiation and lymph node metastasis (5,6,20). In patients with NSCLC, reduced E-cadherin expression has been reported to be associated with tumor dedifferentiation, lymph node metastasis and a poor prognosis (11~16). However, our study revealed no significant relationships between the reduced E-cadherin expression and most of the clinicopathological parameters, such as histological grade and TNM stage, and also the survival, which may be generally useful as prognostic parameters. Similar negative results were also found in some other studies, which showed that the lack of the cadherin- catenin complex expression could not be correlated to any histopathological criteria of epithelial carcinomas (21). Nawrocki et al. explained this by their findings that a correlation between negative regulation of adhesion molecule expression and tumor progression is not always found in vivo, depending on the type of cancer studied and the detection methods used (22). Also, this negative correlation might emphasize the complexity of the cell-adhesion system and the importance of examining more than just the E-cadherin expression. For example, it has been documented that abnormal cell-cell adhesion can exist in tumor cell lines deficient in α-catenin, despite these cells having normal amounts of E-cadherin on their surfaces (23).
This study showed a significant correlation between E-cadherin expression and the NSCLC histological type. The squamous cell carcinomas revealed a significant decrease of E-cadherin expression compared to the adenocarcinomas (p=0.032). This result was consistent with those of the studies by Lee et al. and Choi et al. (11,18). According to other studies, the authors suggested that squamous cell carcinomas had a different pattern of E-cadherin expression from that of adenocarcinomas (19). Therefore, our result might suggest that the examination of E-cadherin expression in the NSCLC could be helpful in the differential diagnosis between squamous cell carcinomas and adenocarcinomas.
The mechanism of correlation between E-cadherin expression and smoking habit or men is unclear. It might be explained by the fact that the main histological type of lung cancer in male smokers is a squamous cell carcinoma, which has relatively lower E-cadherin expression.
It was found that reduced or absent E-cadherin expression was weakly associated with high cell proliferation activity, as measured by Ki-67 staining. It has been postulated that as a result of decreased E-cadherin expression, there is a loss of negative feedback control, which is determined by a cell-cell interaction that results in increased proliferation of cells (24). Also, it has recently become clear that E-cadherin also has a growth suppressor function, inducing cell cycle arrest via upregulation of the cyclin-dependent kinase inhibitor, p27 (25).
In summary, the E-cadherin expression was reduced in the vast majority of NSCLC (78.4%) and particularly in the squamous cell carcinoma histological type, which is known to be the most common type of lung cancer in male smokers. Although the expression was not associated with any other significant clinicopathological factors or the survival, the tumors with reduced E-cadherin expression showed a weak relationship with high proliferative activity, as measured by Ki-67 labeling.
Go to : 
References
1. Smythe WR, Williams JP, Wheelock MJ, Johnson KR, Kaiser LR, Albelda SM. Cadherin and catenin expression in normal human bronchialepithelium and non-small cell lung cancer. Lung Cancer. 1999; 24:157–168. PMID: 10460003.
2. Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991; 251:1451–1455. PMID: 2006419.

3. Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993; 5:806–811. PMID: 8240824.

4. Nakanishi Y, Ochiai A, Akimoto S, Kato H, Watanabe H, Tachimori Y, Yamamoto S, Hirohashi S. Expression of E-cadherin, α-catenin, β-catenin and plakoglobin in esophageal carcinomas and its prognostic significance: immunohistochemical analysis of 96 lesions. Oncology. 1997; 54:158–165. PMID: 9075789.
5. Gabbert HE, Mueller W, Schneiders A, Meier S, Moll R, Birchmeier W, Hommel G. Prognostic value of E-cadherin expression in 413 gastric carcinomas. Int J Cancer. 1996; 69:184–189. PMID: 8682585.

6. Dorudi S, Hanby AM, Poulsom R, Northover J, Hart IR. Level of expression of E-cadherin mRNA in colorectal cancer correlates with clinical outcome. Br J Cancer. 1995; 71:614–616. PMID: 7880746.

7. Ihara A, Koizumi H, hashizume R, Uchikoshi T. Expression of epithelial cadherin and α- and β-catenin in nontumoral livers and hepatocellular carcinomas. Hepatology. 1996; 23:1441–1447. PMID: 8675162.
8. Gunji N, Oda T, Todoroki T, Kanazawa N, Kawamoto T, Yuzawa K, Scarpa A, Fukao K. Pancreatic carcinoma: correlation between E-cadherin and α-catenin expression status and liver metastasis. Cancer (Phila.). 1998; 82:1649–1656. PMID: 9576284.
9. Cheng L, Nagabhushan M, Pretlow TP, Amini SB, Pretlow TG. Expression of E-cadherin in primary and metastatic prostate cancer. Am J Pathol. 1996; 148:1375–1380. PMID: 8623909.
10. Fei Q, Zhang H, Chen X, Wang J-C, Zhang R, Xu W, Zhang Z, Zou W, Zhang K, Qi Q, Wang M, Tao S, Luo Z. Defected expression of E-cadherin in non-small cell lung cancer. Lung Cancer. 2002; 37:147–152. PMID: 12140137.

11. Lee Y-C, Wu C-T, Chen C-S, Chang Y-L. E-cadherin expression in surgically-resected non-small cell lung cancers-A clinicopathological study. Thorac Cardiovasc Surg. 2000; 48:294–299. PMID: 11100763.
12. Kase S, Sugio K, Yamazaki K, Okamoto T, Yano T, Sugimachi K. Expression of E-cadherin and β-catenin in human non-small cell lung cancer and the clinical significance. Clin Cancer Res. 2000; 6:4789–4796. PMID: 11156236.
13. Lim S-C, Jang I-G, Kim Y-C, Park K-O. The role of E-cadherin expression in non-small cell lung cancer. J Korean Med Sci. 2000; 15:501–506. PMID: 11068984.

14. Liu D, Huang C-l, Kameyama K, Hayashi E, Yamauchi A, Koabyashi S, Yokomise H. E-cadherin expression associated with differentiation and prognosis in patients with non-small cell lung cancer. Ann Thorac Surg. 2001; 71:949–955. PMID: 11269479.

15. Sulzer MA, Leers MPG, van Noord JA, Bollen ECM, Theunissen PHMH. Reduced E-cadherin expression is associated with increased lymph node metastasis and unfavorable prognosis in non-small cell lung cancer. Am J Respir Crit Care Med. 1998; 157:1319–1323. PMID: 9563756.

16. Shibanuma H, Hirano T, Tsuji K, QingFang W, Shrestha B, Konaka C, Ebihara Y, Kato H. Influence of E-cadherin dysfunction upon local invasion and metastasis in non-small cell lung cancer. Lung Cancer. 1998; 22:85–95. PMID: 10022216.

17. Bohm M, Totzeck B, Wieland I. Differences of E-cadherin expression levels and patterns in human lung cancer. Ann Hematol. 1994; 68:81–83. PMID: 7511935.

18. Choi YS, Shim YM, Kim S-H, Son DS, Lee H-S, Kim GY, Han J, Kim J. Prognostic significance of E-cadherin and β-catenin in resected stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2003; 24:441–449. PMID: 12965318.
19. Bohm M, Totzeck B, Birchmeier W, Wieland I. Differences of E-cadherin expression levels and patterns in primary and metastatic human lung cancer. Clin Exp Metastasis. 1994; 12:55–62. PMID: 8287621.
20. Oh BR, Nah GJ, Kim SJ, Sim JH, Kwon DD, Park KS, Ryu SB, Park YI. The clinical usefulness of membranous E-cadherin in transitional cell carcinoma of the bladder. J Korean Cancer Assoc. 1998; 30:1219–1226.
21. Han AC, Peralta-Soler A, Knudsen KA, Wheelock MJ, Johnson KR, Salazar H. Differential expression of N-cadherin in pleural mesotheliomas and E-cadherin in lung adenocarcinomas in formalin-fixed, paraffin-embedded tissues. Hum Pathol. 1997; 28:641–645. PMID: 9190996.

22. Nawrocki B, Polette M, Van Hengel J, Tournier J-M, Van Roy F, Birembaut P. Cytoplasmic redistribution of E-cadherin-catenin adhesion complex is associated with down-regulated tyrosine phosphorylation of E-cadherin in human bronchopulmonary carcinomas. Am J Pathol. 1998; 153:1521–1530. PMID: 9811344.

23. Morton RA, Ewing CM, Nagafuchi A, Tsukita S, Issacs WB. Recution of E-cadherin levels and deletion of the α-catenin gene in human prostate cancer cells. Cancer Res. 1993; 53:3585–3590. PMID: 8339265.
24. Janowski JA, Newham PM, Kandemir O, Hirano M, Takeichi M, Pignatelli M. Differential expression of E-cadherin in normal, metaplastic and dysplastic oesophageal mucosa: a putative biomarker. Int J Oncol. 1993; 4:441–448.
25. St Croix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS. E-cadherin dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27 (KIP1). J Cell Biol. 1998; 142:557–571. PMID: 9679152.
Go to : 




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download