Abstract
Purpose
Adriamycin® is one of the most commonly used drugs in the treatment of breast cancer. This study was performed to understand the molecular mechanisms of drug resistance in breast cancer cells.
Materials and Methods
We have analyzed the MCF-7 breast cell line and its adriamycin-resistant variants, MCF-7/ADR using human 10 K element cDNA microarrays.
Results
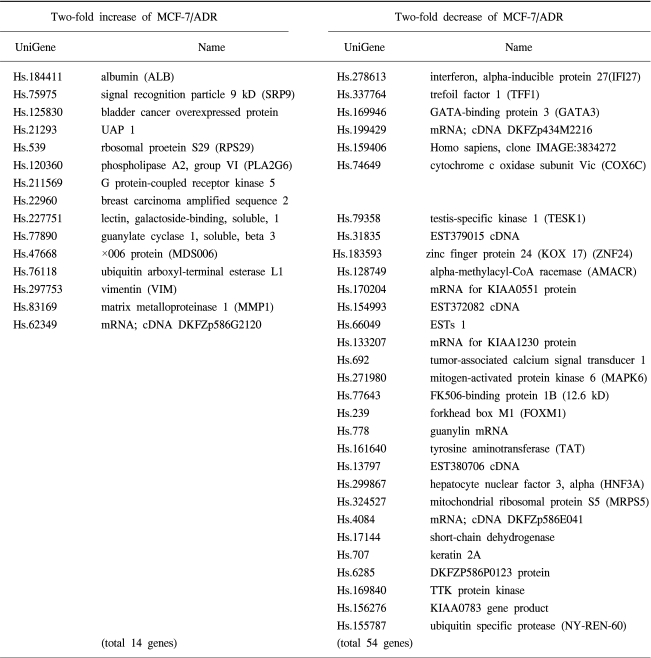
We defined 68 genes that were up-regulated (14 genes) or down-regulated (54 genes) in adriamycin resistant breast cancer cells. Several genes, such as G protein-coupled receptor kinase 5, phospholipase A2, guanylate cyclase 1, vimentin, matrix metalloproteinase 1 are up-regulated in drug resistant cells. Several genes, such as interferon, alpha-inducible protein 27, forkhead box M1, mitogen-activated protein kinase 6, regulator of mitotic spindle assembly 1 and tumor necrosis factor superfamily are down-regulated in adriamycin resistant cells. The altered expression of genes observed in microarray was verified by RT-PCR.
Conclusion
These findings show that cDNA microarray analysis can be used to obtain gene expression profiles reflecting the effect of anticancer drugs on breast cancer cells. Such data may lead to the assigning of signature expression profiles of drug-resistant tumors which may help predict responses to drugs and assist in the design of tailored therapeutic regimens to overcome drug resistance.
Drug resistance in cancer is a major limitation for successful chemotherapy. Adriamycin is one of the most commonly used drugs in the treatment of breast cancer. It has been reported that tumor cell resistance to adriamycin is associated with altered expression of topoisomerase II (1) and integrins (2), changes in glutathione level (3), and expression of membrane-associated pumps such as P-glycoprotein encoded by the multidrug resistance gene MDR1 (4). However, mechanisms of development of intrinsic drug resistance are not thoroughly understood and may involve the expression of multiple genes during tumor progression and also the emergence of acquired resistance to chemotherapeutic agents may be associated with cancer cell selection during chemotherapy. The DNA microarray technology has enabled the analysis of the expression profiles of tumor cell of thousands of genes simultaneously (5,6). To understand the molecular mechanisms of drug resistance in breast cancer cells, we have analyzed the MCF-7 breast cell line and its adriamycin-resistant variants, MCF-7/ADR, using human 10K element cDNA microarrays to identify differential gene expression.
The human breast cancer cell lines, MCF-7 were cultured in 10% fetal bovine serum (FBS) RPMI media containing 100 U/ml penicillin, 100 µg/ml streptomycin and 20 mM glutamine with 5% CO2 at 37℃ in humidified air. Adriamycin resistance cells, MCF-7/ADR were kindly obtained from Dr. Park YM at Incheon University and MCF-7/ADR cells showed significant resistance to adriamycin compared to parent MCF-7 cells (comparative cell viabilities assessed by MTT assay after 36 hour incubation with adriamycin, MCF-7: MCF-7/ADR=100%: 210%).
The experiments were performed on Human 10 K cDNA microarrays (GaiaGene Inc., Seoul, Korea). Human 10 K cDNA microarray consists of 10,336 spots including UniGEM-V clones (Incyte Pharmaceuticals, Palo Allo, CA), housekeeping genes, and yeast DNAs as negative control. UniGEM-V clones contain 9,182 unique and sequence verified cDNA elements mapped to 8,353 UniGene Homo sapiens annotated clusters (7).
Total RNA was extracted using the Trizol reagent (Life Technologies, Grand Island, NY). For labeling of probes with amino allyl cDNA labeling kit, we proceeded as follows: 20 µg of total RNA (12 µl) were combined with oligo (dT) primer (50 uM, 1 µl). The mix was incubated at 70℃ for 10 min, and then keep at room temperature. Primer/RNA solution was added to the RT mix (10X RT buffer, 2 µl; RNase inhibitor, 10 units/µl, 1 µl; dNTP mix (10 mM dATP, dCTP, and dGTP), 1 µl; AAdUTP mix (3 mM dTTP and 3 mM amino allyl dUTP), 1 µl; M-MLV reverse transcriptase, 200 units/µl, 2 µl) and incubated at 42℃ for 2 hours. The RNA was hydrolyzed with NaOH (1 M, 4 µl) at 65℃ for 15 min. The solution was neutralized with HEPES (1 M, 10 µl) and the cDNA was recovered by ethanol precipitation using sodium acetate (3 M, 3.4 µl) and ethanol (100%, 100 µl). The cDNA pellet was resuspended in 4.5 µl of coupling buffer and added 2.5 µl of nuclease-free water and 3 µl of dye (NHS-ester Cy3 or Cy5, 2.22 mM in DMSO). The resulting solution was mixed by gentle vortexing and centrifuged briefly. The tubes were wrapped in aluminum foil, and incubated at room temperature for one hour in a dark room. The labeling reaction was stopped with 6 µl of 4 M hydroxylamine hydrochloride. And the tubes were vortexed, briefly centrifuged, and incubated for 15 min at room temperature in the dark. The solution was added 70 µl of nuclease-free water, then NucAway spin columns were used to purify the dye labeled cDNA. The labeled cDNA was pelleted by ethanol precipitation and then resuspended in 10 µl of nuclease-free water.
Prehybridization of cDNA microarray was done at 55℃ for 1 hour with prehybridization solution (5×SSC, 0.1% SDS, 1% bovine serum albumin) in the 50 ml conical tube. The Cy3- and Cy5-labeled products were combined, and to this 8 µg of poly (dA) (Pharmacia, Kalanzoo, MI), 4 µg of Escherichia coli tRNA (Sigma Chemical, St. Louis, MD), 10 µg of Human Cot1 DNA (Life Technologies, Grand Island, NY), and EasyHyb hybridization solution (U-Vision Biotech, Taiwan) were added to a final volume of 60 µl. The probe was) at 100℃ for 2 min, cleared a debris by centrifugation, and placed onto the glass slide with a coverslip in a hybridization chamber (TeleChem International, Sannyvale, CA). Hybridization was performed in a 55℃ water bath for 12 to 16 hours. The slides were subsequently washed in 2×SSC, 0.2% SDS at room temperature for 5 min, 0.1×SSC, 0.2% SDS at 55℃ for 5 min, after a quick rinse in 0.1×SSC. The slides were spun dry (500 rpm) for 5 min.
The two fluorescent images (Cy3 and Cy5) were scanned separately from a GMS 418 Array Scanner (Affymetrix, Santa Clara, CA). The images were analyzed using MAAS program (GaiaGene; http//www.gaiagene.com). To determine the background signal intensity, we spotted the yeast DNA on each slide. Values at least twofold above the background intensity were considered significant. To filter out the unreliable data, spots with signal-to-noise ratios below three were not included in the data. Each experiment was performed three times and scatterplot showed consistent findings among each experiment (data not shown).
From each sample, 5 µg of total RNA were reverse-transcribed for single-stranded cDNAs using 1 mM of oligo-dT primer with Superscript II reverse transcriptase (Life Technologies, Grand Island, NY). Each single-stranded cDNA was diluted for subsequent PCR amplification by monitoring GAPDH as a quantitative control. Each PCR was carried out in a 50 µl volume of 1×PCR buffer for 5 minutes at 95℃ for initial denaturing, followed by 25~35 cycles of 95℃ for 45 seconds, 56℃ for 45 seconds, and 72℃ for 1 minutes, in the Perkin-Elmer Cycler 9,600 (Perkin-Elmer Applied Biosystems, Foster, CA). The sequences of the primers used for RT-PCR were as follows: β-actin forward, 5'-TGACGGGGTCACCCACACTGTGCCCATCTA-3', reverse, 5'-CTAGAAGCATTGCGGTGGACGATGGAGGG-3'; interferon, alpha-inducible protein 27 forward, 5'-TCTGGCTCTGCCGTAGTTTT-3', reverse, 5'-GGCATGGTTCTCTTCTCTGC-3'; trefoil factor 1 forward, 5'-AACACACTCCTGGGGATCAG-3', reverse, 5'-AGCCTGATGAAGCTGGAAAA-3'; GATA-binding protein 3 forward, 5'-TGGCAGTTTGTCCATTTGAA-3', reverse, 5'-TTCGACTTGCATTTTTGCAG-3'; G protein-coupled receptor kinase 5 forward, 5'-AATGGAGCTGGAAAACATCG-3', reverse, 5'-GCCAACTTGGGCTATGAAAA-3'; x006 protein (MDS006) forward, 5'-CATCCTGAGACCATGCCTTC-3', reverse, 5'-GCCTCACAATCACCACCTTT-3', matrix metalloproteinase 1 forward, 5'-ATGCTGAAACCCTGAAGGTG-3', reverse, 5'-CTGGTTGAAAAGCATGAGCA-3'.
cDNAs obtained from adriamycin-sensitive or -resistant breast cancer cells were differentially labeled with Cy5 and Cy3, respectively and hybridized to the 10,336 spotted gene microarray. To filter out the unreliable data and identify genes with significantly different expression, two cutoff analysis methods were carried out. Spots with signal-to-noise ratio below 3 and with less signal intensity than negative spots were not investigated any further. After cutoff, we selected genes with expression ratios of 2-fold up and down regulated.
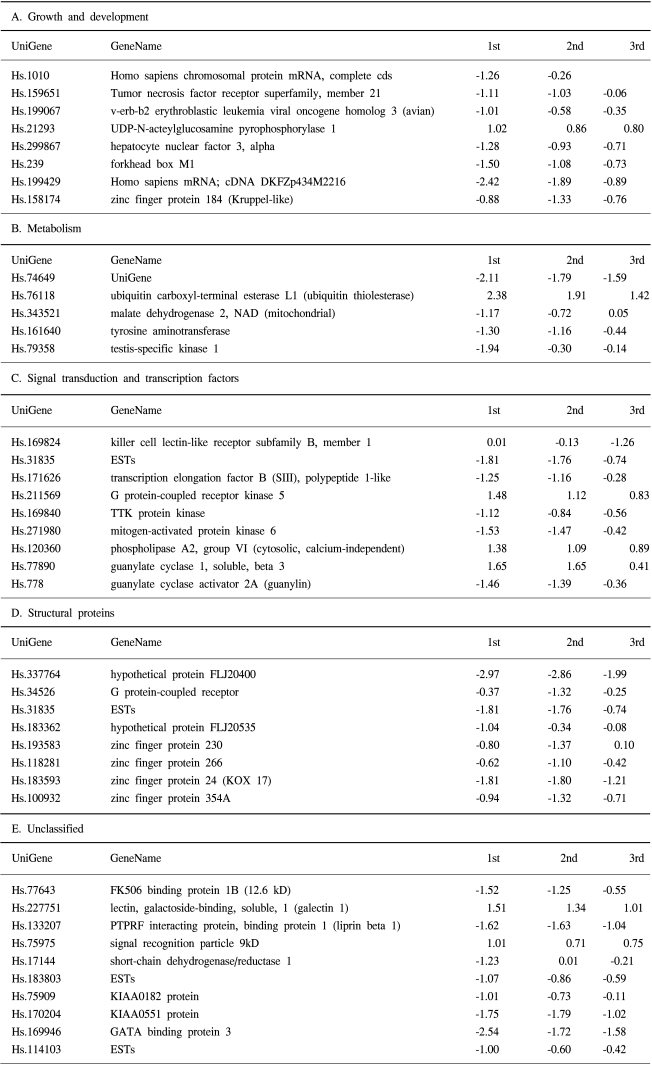
We defined 68 genes that were up-regulated (14 genes) or down-regulated (54 genes) in adriamycin-resistant breast cancer cells (Table 1). Several genes, such as G protein-coupled receptor kinase 5, phospholipase A2, guanylate cyclase 1, vimentin, matrix metalloproteinase 1 are up-regulated in adriamycin-resistant cells. Several genes, such as interferon, alpha-inducible protein 27, forkhead box M1, mitogen-activated protein kinase 6, regulator of mitotic spindle assembly 1 and tumor necrosis factor superfamily are down-regulated in adriamycin-resistant cells. The genes were classified into five categories according to their function and pathways (Table 2).
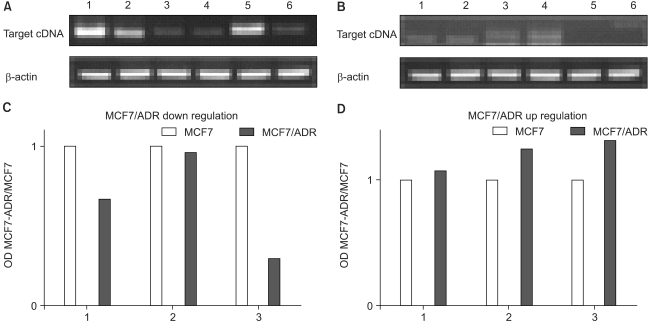
We subsequently performed semiquantitative RT-PCR with a subset of differentially expressed cDNAs to confirm the observed differential gene expression. We chose three up-regulated genes (G protein-couples receptor kinase 5, ×006 protine (MDS006), and matrix metalloproteinase) and three down-regulated genes (interferon, alpha-inducible protein 27, trefoil factor 1, and GATA-binding protein 3) from adriamycin-sensitive and -resistant breast cancer cells for RT-PCR confirmation (Fig. 1). The results of these validations demonstrated the very similar expression pattern to that of the microarray analyses. In general, for a given mRNA, the cDNA microarray data and the results from the RT-PCR studies are in agreement.
Emergence of drug resistance in breast cancer is a major obstacle to successful chemotherapy. In this study we used cDNA microarray technology to examine gene expression profiles in adriamycin-sensitive or -resistant breast cancer cells. We defined 68 genes that were up-regulated (14 genes) or down-regulated (54 genes) in adriamycin-resistant breast cancer cells and could be classified into five categories according to their function and pathways. This list included growth and development, metabolism, signal transduction and transcription factors, and structural proteins.
The most prominent finding was that a gene, cytochrome c oxidase which has been known to be involved in mitochondrial modifications. Cytochrome c oxidase was down-regulated in adriamycin-resistant breast cancer cells. Cytochrome c oxidase is a terminal enzyme of the mitochondrial respiratory chain and catalyzes the transfer of electrons from cytochrome c to molecular oxygen (8~10). Dysfunction of the mitochondrial respiratory chain has been associated with apoptosis induction (11). One of the cytotoxic mechanisms of adriamycin is to inhibit cytochrome c oxidase activity (12). It also has been reported that adriamycin-resistant leukemia K562 cells showed a lower expression of catalytic subunits cytochrome c oxidase subunit (13,14). In this study, adriamycin-resistant breast cancer cells also showed lower expression of cytochrome c oxidase suggesting the alteration of this enzyme level in mitochondria may play a key role in the mechanism of resistance to adriamycin. We also found that other mitochondrial proteins such as, mitochondrial ribosomal protein S5 and tyrosine aminotransferase, nuclear gene encoding mitochondrial protein, were also down-regulated in adriamycin-resistant cells.
Another interesting finding was the down-regulated expression of tumor necrosis factor receptor superfamily. Apoptosis is a fundamental mechanism of cell death that can be engaged by a range of cellular insults. One of the major modes of cancer cell killing by chemotherapeutic drugs is executed via the activation of apoptosis. Anti-cancer drugs and cytotoxic cytokines such as members of the TNF/Fas-ligand family play a predominant role in apoptosis induction in tumor cells, and are critical in cancer therapy (15,16). Apoptosis may involve the activation of death-inducing ligand/receptor systems such as CD95 (APO-1/Fas) and treatment with cytotoxic drugs may result in up-regulation of CD95, thereby increasing the sensitivity to the CD95 death signal (17,18). In addition, deficient activation of the CD95 system was found in drug-resistant cells suggesting that the lower expression of tumor necrosis factor receptor superfamily might be associated with drug resistance.
In adriamycin-resistant breast cells, several genes involved in cell adhesion and invasiveness such as matrix metalloproteinase-like 1 and vimentin were up-regulated and these results were confirmed by RT-PCR. Matrix metalloproteinase 1 is involved in metastasis and invasion, and cells with multidrug resistant phenotype have been previously reported to have an increased invasive ability. The increased expression of matrix metalloproteinase 1 was consistent with this recognized characteristics (19). Recently, it was also reported that the expression of matrix metalloproteinase 1 was increased in both intrinsic and acquired doxorubicin-resistant breast carcinoma cells by cDNA microarray (20). One mechanism whereby matrix metalloproteinase 1 may promote tumor survival and resistance to doxorubicin is by cleaving FasL and reducing its effectiveness in trigerring Fas-mediated apoptosis (21).
Another gene increased in expression in adriamycin-resistant breast cancer cells was vimentin. Epithelium-derived cancer cells do not normally express detectable levels of vimentin (22), whereas previous studies reported that in breast, colon and ovarian carcinoma cell lines resistance to adriamycin is frequently accompanied by overexpression of this intermediate filament protein (23,24). It has been suggested that vimentin overexpression in human breast carcinoma cells is not causally related to drug resistance because cells transfected with human vimentin did not become resistant, indicating that overexpression of vimentin is associated with increasingly malignant properties, including loss of estrogen receptors and invasiveness (24).
Although in this study the expression profiles of MCF-7 and established adriamycin-resistant MCF-7 cells were compared, it has been reported that a subset of genes expressed after transient exposure to adriamycin was also constitutively overexpressed in the adriamycin-resistant cell line. This distinct set of overlapping genes may represent the signature profile of adriamycin-induced gene expression and resistance in cancer cells (25).
We have used microarrays to identify a differential gene expression pattern associated with adriamycin-resistant breast cancer cells and found several small sets of genes were up- or down-regulated. Such a gene expression pattern would provide useful prognostic markers of predicting emergence of drug resistance during chemotherapy. Some of the genes proved here in the adriamycin-resistant breast cancer cells could be promising candidates for chemotherapeutic targets in treatment of adriamycin-resistant carcinomas.
References
1. Nielsen D, Maare C, Skovsgaard T. Cellular resistance to anthracyclines. Gen Pharmacol. 1996; 27:251–255. PMID: 8919638.

2. Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999; 93:1658–1667. PMID: 10029595.

3. Sinha BK, Mimnaugh EG, Rajagopalan S, Myers CE. Adriamycin activation and oxygen free radical formation in human breast tumor cells: protective role of glutathione peroxidase in adriamycin resistance. Cancer Res. 1989; 49:3844–3848. PMID: 2544260.
4. Dalton WS. Mechanisms of drug resistance in hematologic malignancies. Semin Hematol. 1997; 34(Suppl 5):3–8. PMID: 9408955.
5. DeRisi J, Penland L, Brown PO, Bittner ML, Meltzer PS, Ray M, Chen Y, Su YA, Trent JM. Use of cDNA microarray to analyze gene expression patterns in human cancer. Nat Genet. 1996; 14:457–460. PMID: 8944026.
6. Yoon JH, Lee JM, Namkoong SE, Bae SM, Kim YW, Han SJ, Cho YL, Nam GH, Kim GK, Seo JS, Ahn WS. cDNA microarray analysis of gene expression profiles associated with cervical cancer. Cancer Res Treat. 2003; 35:451–459.

7. Lee KH, Chang MY, Ahn JI, Yu DH, Jung SS, Choi JH, Noh YH, Lee YS, Ahn MJ. Differential gene expression in retinoic acid-induced differentiation of acute promyelocyteic leukemia cells, NB4 and HL-60 cells. Biochem Biophys Res Commun. 2002; 296:1125–1133. PMID: 12207890.
8. Kadenbach B, Jarausch J, Hartmann R, Merle P. Separation of mammalian cytochrome c oxidase into 13 polypeptides by a sodium dodecyl sulfate-gel electrophoretic procedure. Anal Biochem. 1983; 129:517–521. PMID: 6303162.

9. Capaldi RA. Structure and function of cytochrome c oxidase. Annu Rev Biochem. 1990; 59:569–596. PMID: 2165384.

10. Taanman JW. Human cytochrome c oxidase: structure, function and deficiency. J Bioenerg Biomembr. 1997; 29:151–163. PMID: 9239540.
11. Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000; 10:369–377. PMID: 10932094.

12. Muller I, Niethammer D, Bruchelt G. Anthracycline-derived chemotherapeutics in apoptosis and free radical cytotoxicity. Int J Mol Med. 1998; 1:491–494. PMID: 9852255.
13. Denis-Gay M, Petit JM, Mazat JP, Ratinaud MH. Modifications of oxido-reductase activities in adriamycin-resistant leukemia K562 cells. Biochem Pharmacol. 1998; 56:451–457. PMID: 9763220.
14. Grandjean F, Bremaud L, Robert J, Ratinaud MH. Alterations in the expression of cytochrome c oxidase subunits in doxorubicin-resistant leukemia K562 cells. Biochem Pharmacol. 2002; 63:823–831. PMID: 11911833.

15. Ashkenazi A. Targeting death and decoy receptors of the tumor-necrosis factor superfamily. Nat Rev Cancer. 2002; 2:420–430. PMID: 12189384.
16. Inoue S, Salah-Eldin AE, Omoteyama K. Apoptosis and anticancer drug resistance. Hum Cell. 2001; 14:211–221. PMID: 11774740.
17. Friesen C, Fulda S, Debatin KM. Cytotoxic drugs and the CD95 pathway. Leukemia. 1999; 13:1854–1858. PMID: 10557062.

18. Watts GS, Bernard W, Futscher BW, Isett R, Gleason-Guzman M, Kunkel MW, Salmon SE. cDNA microarray analysis of multidrug resistance: doxorubicin selection produces multiple defects in apoptosis signaling pathways. J Pharmacol Exp Ther. 2001; 299:434–441. PMID: 11602652.
19. Weinstein RS, Jakate SM, Dominguez JM, Lebovitz MD, Koukoulis GK, Kuszak JR, Klusens LF, Grogan TM, Saclarides TJ, Roninson IB, Coon JS. Relationship of the expression of the multidrug resistance gene product (P-glycoprotein) in human colon carcinoma to local tumor aggressiveness and lymph node metastasis. Cancer Res. 1991; 51:2720–2726. PMID: 1673639.
20. Turton NJ, Judah DJ, Riley J, Davies R, Lipson D, Styles JA, Smith AG, Gant TW. Gene expression and amplification in breast carcinoma cells with intrinsic and acquired doxorubicin resistance. Oncogene. 2001; 20:1300–1306. PMID: 11313874.

21. Misiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001; 61:577–581. PMID: 11212252.
22. Steinert PM, Roop DR. Molecular and cellular biology of intermediate filaments. Ann Rev Biochem. 1988; 57:593–625. PMID: 3052284.

23. Sommers CL, Heckford SE, Skerker JM, Worland P, Torri JA, Thompson EW, Byers SW, Gelmann EP. Loss of epithelial markers and acquisition of vimentin expression in adriamycin- and vinblastine-resistant haman breast cancer cell lines. Cancer Res. 1992; 52:5190–5197. PMID: 1382837.
24. Conforti G, Codegoni AM, Scanziani E, Dolfini E, Dasdia T, Calzza M, Caniatti M, Broggini M. Different vimentin expression in two clones derived from a human colocarcinoma cell line (LoVo) showing different sensitivity to doxorubicin. Br J Cancer. 1995; 71:505–511. PMID: 7880731.

25. Kudoh K, Ramanna M, Ravatn R, Elkahoun AG, Bittner ML, Meltzer PS, Trent JM, Dalton WS, Chin KV. Monitoring the expression profiles of doxorubicin-induced and doxorubicin-resistant cancer cells by cDNA microarray. Cancer Res. 2000; 60:4161–4166. PMID: 10945624.
Fig. 1
Verfication of cDNA microarray data by semi-quantitative PCR. A: 1.MCF7 (IFI27), 2.MCF7/ADR (IFI27), 3.MCF7 (TFF1), 4.MCF7/ADR (TFF1), 5.MCF7 (GATA3), 6.MCF7/ADR (GATA3). B: 1.MCF7(GPRK5), 2.MCF7/ADR (GPRK5), 3.MCF7 (GYCY1B3), 4.MCF7/ADR (GYCY1B3), 5.MCF7 (MMP1), 6.MCF7/ADR (MMP1). C: 1. black-MCF7 (IFI27), gray-MCF7/ADR (IFI27), 2.black-MCF7 (TFF1), gray-MCF7/ADR (TFF1), 3. black-MCF7 (GATA3), gray-MCF7/ADR (GATA3) .D: 1.black-MCF7 (GPRK5), gray-MCF7/ADR (GPRK5), 2. black-MCF7 (GYCY1B3), gray-MCF7/ADR(GYCY1B3), 3. black-MCF7 (MMP1), gray-MCF7/ADR (MMP1) IFI27, interferon, alpha-inducible protein 27; TFF, trefoil factor 1; GATA3, GATA-binding protein 3; GPRK5, G protein-coupled receptor kinase 5; GYCY1B3, ×006 protein (MDS006); MMP1, matrix metalloproteinase 1 *OD MCF7-ADR/MCF7 demonstrated relative OD ratio for each MCF7-ADR and MCF7.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download