Abstract
Purpose
92 kDa matrix metalloproteinase-9 (MMP-9) is believed to play an important role in degrading the matrix and basement membrane, contributing to the invasion and metastasis of malignant solid tumors. However, little is known about its involvement in a malignant fibrous histiocytoma. The aim of this study was to investigate the expression of MMP-9 and to correlate its expression with clinicopathologic parameters in human malignant fibrous histiocytomas.
Materials and Methods
Archival tumor tissues from 20 patients with a malignant fibrous histiocytoma were analyzed by immunohistochemistry for the expression of MMP-9. Clinical information was obtained through the computerized retrospective database from the tumor registry.
Sarcomas are very rare, accounting for approximately 1% of all malignancies. They are a heterogeneous group of solid tumors with multiple histologic subtypes, varying biological characteristics, and differing clinical behaviors (1). Pleomorphic soft tissue sarcomas without specific differentiation have been termed malignant fibrous histiocytomas (MFHs). MFHs are the most common soft tissue sarcomas of late adult life (2). With only limited data, the biological potential of this neoplasm may not yet be fully understood. Unfortunately, the prognosis of pleomorphic sarcomas is generally poor and it was found that 40% of patients with a high-grade MFH die of disease within 5 years (3). It is thus important to identify factors that may aid in predicting patients' survival.
Matrix metalloproteinases (MMPs) are a family of zinc-dependent proteinases that mediate the degradation of the extracellular matrix, which is important for tumor cell invasion and metastasis (4). These enzymes have been classified into various groups depending on their substrates, including collagenases, gelatinases, stromelysins, membrane type, and non-classified (5). They are absent or expressed at very low levels in normal tissues of the host, whereas they have been found to be upregulated in malignant tissues (6). The gelatinase subgroup of MMPs includes 72-kDa gelatinase (MMP-2) and 92-kDa gelatinase (MMP-9). MMP-9 contains fibronectin-like domains for collagen binding and is capable of degrading type I, IV, V, VII, and XI collagens and laminin. Such proteolytic ability suggests that MMP-9 ultimately regulates cell migration, tumor growth, and angiogenesis (7). It has been reported to be overexpressed in many human malignancies including solid tumors and hematologic neoplasms (8,9). Furthermore, an in vitro overexpression of MMP-9 confers a metastatic phenotype (10).
The current literature, however, contains very limited data regarding MMP-9 in soft tissue sarcomas, especially in MFHs. In the present study, we analyzed a group of MFHs and assessed the MMP-9 expression. We then correlated the findings with the clinical outcomes.
Twenty soft tissue sarcomas originally diagnosed as MFHs using light and electron microscopy were retrieved from the files of our institution. All patients were surgically treated between January, 1995 and December, 2002. Tumors were graded using the French (FNCLCC) system (11). After an initial review of all available hematoxylin and eosin-stained slides of the surgical specimens to confirm the diagnosis, we selected one representative paraffin block from each case for further study. Clinical information was obtained through a computerized retrospective database of the tumor registry. The survival times of the patients were calculated from the date of surgery. The follow-up was conducted until August, 2004 for a mean of 39 months.
Immunohistochemical studies were performed using the streptoavidin-biotin method, as previously described (12). Briefly, 4-µm thick sections were cut from each block and immunostained for MMP-9 (Zymed, San Francisco, CA). MMP-9 was raised against intact human MMP-9, recognizing both the latent and active forms of the enzyme. Deparaffinized and hydrated tissue sections were immersed in methanol containing 0.03% H2O2 for 30 min to block endogenous peroxidase activity. All of the sections were treated with 0.1 N citrate buffer for 5 min to retrieve the unmasked antigens. Then the sections were incubated in normal horse serum (diluted 1:20) for 30 min to block the nonspecific antibody binding sites. The monoclonal antibody for MMP-9 was diluted in phosphate-buffered saline supplemented with 5% normal horse serum and 1% bovine serum albumin at a dilution ratio of 1:200. The sections were incubated with the primary monoclonal antibody at 4℃ overnight. The sections were treated with biotinylated anti-mouse IgG horse serum for 30 min and avidin DH-biotinylated horseradish peroxidase complexes for 30 min. Afterwards, the sections were colored with 3,3'-diaminobezidine tetrahydrochloride for 10 min, and then counterstained with 1% Mayer's hematoxylin.
All the sections stained by immunohistochemistry were scored separately by two authors to exclude interobserver variability. Only cytoplasmic staining was regarded as positive for MMP-9. The sections were scanned at a low magnification (×40) using light microscopy. Areas with the predominant staining pattern were chosen for further evaluation at a higher magnification (×100). Cases showing immunoreactivity in more than 10% of the tumor cells were interpreted as positive.
Statistical analyses were performed using Pearson's chi-square test and Fisher's exact test for the evaluation of background factors. A P-value less than 0.05 was considered statistically significant. Survival curves were calculated using the Kaplan-Meier method and analyzed by the log rank test.
Clinicopathologic data along with immunohistochemical staining results are summarized in Table 1. Tumor specimens were obtained from 20 patients, including 11 men and 9 women with a mean age of 57.2 years (range 22~74). Ten specimens involved a lower extremity, 4 an upper extremity, 3 a buttock, 2 back and 1 chest wall with a mean size of 9.9 cm (range 1.5~25 cm). Mitotic counts were between 2 and 70 per 10 high power field (mean 21.2). One tumor was poorly differentiated and the remaining 19 were moderately differentiated. Necrosis was present in 7 of the cases: less than 50% in 6 tumors and more than 50% in one tumor. There were 2 grade 1 tumors, 15 grade 2 tumors and 3 grade 3 tumors. Seventeen of the 20 (85%) tumors showed a positive immunoreaction for MMP-9 (Fig. 1). A tumor recurred locally without metastasis in six patients. Eight patients experienced tumor metastasis at a mean interval of 26 months (range 9~51 months) (data not shown).
The relation between prognostic parameters and median survival time is listed in Table 2. There were no associations between the activated MMP-9 expression and patients' age, gender, tumor site and size, mitotic counts, tumor differentiation, necrosis, or tumor grade. The MMP-9 expression also demonstrated no correlation with histologic subtype (data not shown). However, the MMP-9 expression was inversely correlated with patients' survival time (p=.011) (Fig. 2).
To date, few studies on MMP-9 in MFHs have appeared in the literature, although the elevated expression of MMP-2 in fibrohistiocytic tumors is considered to be a common phenomenon (13). The enhanced expression of MMP-2 associated with the activation of MT2-MMP has been demonstrated in MFHs (14). MMPs are produced or secreted by zymogen (pro-MMPs), and are activated by a typical two-step mechanism involving a partially activated intermediate, resulting from an upstream cleavage of a propeptide. In a study of MMP-2 activity in MFHs, it was significantly activated in the tumor cells of all 3 tumors investigated (14). Furthermore, double immunofluorescence labeling demonstrated that the immunoreactive cells with the anti-MMP-2 antibody in all 3 MFHs consistently reacted with anti-MT1-, anti-MT2, and anti-MT3-MMP antibodies, indicating a correlation between the activation of MMP-2 and activated MT-MMPs. The activation of secreted MMP-9 is influenced by the activation of MMP-2 localized in the cellular membrane; furthermore, the secretion and activation of MMP-2 are stimulated by the activation of MT-MMPs, which are unique in their transmembrane domains. More importantly, MMP-9 is known to play a major role in tumor invasion and metastasis (4,10). These findings prompted us to investigate the MMP-9 status in MFHs.
The expression of MMP-9 was analyzed in 20 MFHs, and evaluated for possible correlations with the patients' clinicopathological parameters (age, gender, tumor site and size, mitotic counts, tumor differentiation, necrosis, tumor grade and patient survival). The major findings of our study were as follows: (1) the expression of MMP-9 was observed in 85% of the tumors investigated, and (2) the MMP-9 expression was significantly correlated with patients' poor prognosis.
The understanding of cancer pathogenesis is rapidly growing and evidence indicates that several molecular genetic markers are involved in the initiation and/or progression of the disease. The interactions of the molecular genetic changes are complex; however, some may be important targets for the development of specific inhibitors to serve as potential chemotherapeutic agents. An extracellular matrix and basement membrane turnover is regulated by a delicate balance between the molecular factors that participate in the production, activation, and inhibition of MMPs and the production of natural tissue inhibitors of matrix metalloproteinases (TIMPs) (15). Because of their central role in tumor invasion and metastasis, MMPs are potential targets for therapeutic intervention. Several investigations have demonstrated the effectiveness of anti-MMP therapy. A synthetic small molecule has been shown to inhibit the endotoxin and cytokine-induced synthesis of MMP-9 (16). The inhibition of MMPs such as MMP-9 has produced encouraging results by decreasing the number, size and speed of development of metastatic nodules in animals (17), and has shown activity against metastasis in a rat sarcoma model (18). MMPs as targets for therapy were also described in osteosarcomas and Kaposi sarcomas (19,20). In the present study, the MMP-9 expression was detected in 85% of the samples. The fact that MMP-9 activation is likely to occur in MFHs may make it particularly suitable for treatment with the emerging class of drugs to block MMP-9 secretion and matrix invasion. Using MMP-9 inhibition as one arm of a multitargeted approach will have benefits for the treatment of MFHs, when a larger series of studies confirms MMP-9 representation in these tumors. In addition, considering the many factors involved such as tissue inhibitors of metalloproteinases (TIMPs) or various stimulating factors for MMPs, the relationship between MFHs and TIMPs and other subtypes of MMPs is worthy of further study, which is currently being investigated in our laboratories.
Our data revealed the significance of the MMP-9 expression on a poor prognosis. This is the first time that MMP-9 has been found to be a significant prognostic factor for the survival of patients with an MFH. In the previous studies on MMP-9 in osteosarcomas, patients positive for MMP-9 had a shorter overall survival as in our cases and a high metastatic potential (21,22). In other malignant tumors, an overexpression of MMP-9 is seen in high grade compared with low-grade, and in advanced compared with early-stage tumors (23~25). The expression of MMP-9 in association with angiogenic factors such as the vascular endothelial growth factor has been reported in a variety of human tumors (25). It is most likely that tumor cells positive for MMP-9 may be able to stimulate a new vascular network, contributing to the local recurrence and distant metastasis. Thus, MMP-9 may become a useful prognostic indicator, especially when combined with other factors, including vascularity.
References
1. Pisters PWT, Brennan MF. Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, editors. Sarcomas of soft tissue. Clinical Oncology. 2000. 2nd ed. New York: Churchill Livingstones;p. 2273–2313.
2. Enzinger F, Weiss S. Soft Tissue Tumors. 1995. 3rd ed. St. Louis: Mosby.
3. Pezzi CM, Rawlings MS Jr, Esgro JJ, Pollock RE, Romsdahl MM. Prognostic factors in 227 patients with malignant fibrous histiocytoma. Cancer. 1992; 69:2098–2103. PMID: 1311983.

4. Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor of cell interactions with the extracellular matrix during invasion and metastasis. Ann Rev Cell Biol. 1993; 9:541–573. PMID: 8280471.
5. Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999; 43(Suppl):S42–S51. PMID: 10357558.

6. Scappaticci FA, Marina N. New molecular targets and biological therapies in sarcomas. Cancer Treat Rev. 2001; 27:317–326. PMID: 11908925.

7. Sanceau J, Boyd DD, Seiki M, Bauvois B. Interferons inhibit tumor necrosis factor-α-mediated matrix metalloproteinase-9 activation via interferon regulatory factor-1 binding competition with NF-κB. J Biol Chem. 2002; 277:35766–35775. PMID: 12105194.
8. Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000; 18:1135–1149. PMID: 10694567.

9. Bauvois B, Dumont J, Mathiot C, Kolb JP. Production of matrix metalloproteinase-9 in early stage B-CLL: suppression by interferons. Leukemia. 2002; 16:791–798. PMID: 11986939.

10. Farina AR, Tacconelli A, Vacca A, Maroder M, Gulino A, Mackay AR. Transcriptional up-regulation of MMP-9 expression during spontaneous epithelial to neuroblast phenotype conversion by SK-N-SH neuroblastoma cells, involved in enhanced invasivity, depends upon GT-box and nuclear factor kappaB elements. Cell Growth Differ. 1999; 10:353–367. PMID: 10359016.
11. Coindre JM. Grading in soft tissue sarcomas-how and why? Histopathology. 2002; 41(Suppl 2):236–240. PMID: 12207785.
12. Yoo J, Jung JH, Choi HJ, Kang SJ, Kang CS. The expression of c-myc, bcl-2 and p53 proteins in adenocarcinomas of lung. Cancer Res Treat. 2002; 36:146–150.

13. Soini Y, Salo T, Oikarinen A, Autio-Harmainen H. Expression of 72 and 92 kDa type IV collagenase in malignant fibrous histiocytomas and dermatofibromas. Lab Invest. 1993; 69:305–311. PMID: 8397322.
14. Ohnishi Y, Ito Y, Tajima S, Ishibashi A, Arai K. Immunohistochemical study of membrane type-matrix metalloproteinases (MT-MMPs) and matrix metalloproteinase-2 (MMP-2) in dermatofibroma and malignant fibrous histiocytoma. J Dermatol Sci. 2002; 28:119–125. PMID: 11858950.

15. Reynolds JJ. Collagenases and tissue inhibitor of metalloproteinases: A functional balance in tissue degradation. Oral Dis. 1996; 2:70–76. PMID: 8957940.
16. McMillan JI, Weeks R, West JW, Bursten S, Rice GC, Lovett DH. Pharmacological inhibition of gelatinase B induction and tumor cell invasion. Int J Cancer. 1996; 67:523–531. PMID: 8759612.

17. Brown PD. Parks WC, Mecham RP, editors. Synthetic inhibitors of matrix metalloproteinases. Matrix metalloproteinases. 1998. Sandieqo: Academic Press;p. 243–256.

18. Hua J, Muschel RJ. Inhibition of matrix metalloproteinase-9 expression by a ribozyme blocks metastasis in a rat sarcoma model system. Cancer Res. 1996; 56:5279–5284. PMID: 8912869.
19. Heikkila P, Teronen O, Hirn MY, Sorsa T, Tervahartiala T, Salo T, et al. Inhibition of matrix metalloproteinase-14 in osteosarcoma cells by clodronate. J Surg Res. 2003; 111:45–52. PMID: 12842447.
20. Fingleton B, Matrisian LM. Matrix metalloproteinsases as targets for therapy in Kaposi sarcoma. Curr Opin Oncol. 2001; 13:368–373. PMID: 11555714.
21. Foukas AF, Deshmukh NS, Grimer RJ, Mangham DC, Mangos EG, Taylor S. Stage-IIB osteosarcomas around the knee. A study of MMP-9 in surviving tumour cells. J Bone Joint Surg. 2002; 84:706–711.
22. Himmelstein BP, Asada N, Carlton MR, Collins MH. Matrix metalloproteinase-9 (MMP-9) expression in childhood osseous osteosarcoma. Med Pediat Oncol. 1998; 31:471–474.
23. Rao JS, Yamamoto N, Mohaman S, Gokaslan ZL, Fuller ZN, Stetler-Stevenson WG, et al. Expression and localization of 92-kDa type IV collagenase/gelatinase B (MMP-9) in human gliomas. Clin Exp Metastasis. 1996; 14:12–18. PMID: 8521611.
24. MacDougall JR, Bani MR, Lin Y, Rak J, Kerbel RS. The 92-kDa gelatinase B is expressed by advanced stage melanoma cells: suppression by somatic cell hybridization with early stage melanoma cells. Cancer Res. 1995; 55:4174–4181. PMID: 7664294.
25. Papathoma AS, Petraki C, Grigorakis A, Papakonstantinou H, Karavana V, Stefanakis S, et al. Prognostic significance of matrix metalloproteinases 2 and 9 in bladder cancer. Anticancer Res. 2000; 20:2009–2013. PMID: 10928143.
Fig. 1
Immunoreactivity with MMP-9 was detected in the cytoplasm of most tumor cells. ABC method (×100).
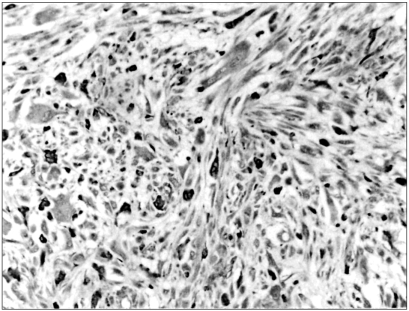
Fig. 2
Kaplan-Meier estimates of patient survivals in cases of MFHs are presented according to the MMP-9 expression.
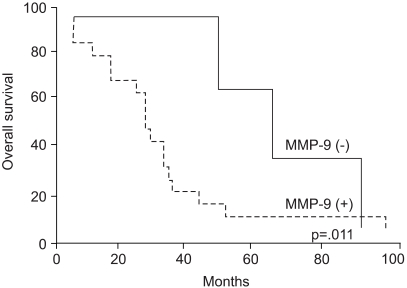
Table 1
Clinicopathologic characteristics
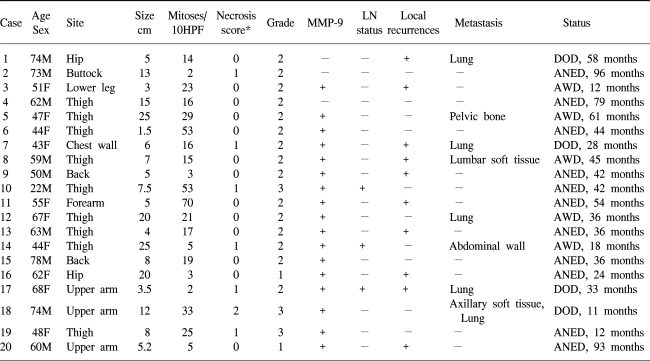
*0=no necrosis, 1=<50% tumor necrosis, 2=≥50% tumor necrosis (ref.11)




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download