Abstract
Objectives
The incidence and risk factors of lymphocele development after pelvic lymphadenectomy were evaluated and its management investigated.
Materials and Methods
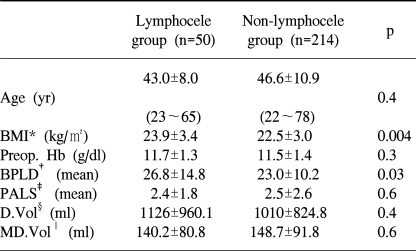
This retrospective study was carried out on 264 patients who received a pelvic lymphadenectomy, between March 1999 and February 2003, due to gynecologic cancer. The patients were classified into two groups; the lymphocele (n=50) and non-lymphocele groups (n=214), as confirmed by ultrasonography, CT scan and MRI. Each group was compared by cancer type and stage, BMI, preoperative Hb, use of pre/postoperative chemotherapy or radiotherapy, number of resected pelvic lymph nodes and the volume of postoperative drainage from a Hemovac® pelvic drain.
Results
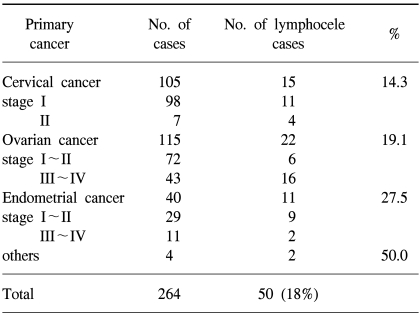
Of the 264 patients tested, 15 of 105 cervical cancer (14%), 22 of 115 ovarian cancer (19%) and 11 of 40 endometrial cancer patients (27%), a total of 50 patients (18%), developed lymphoceles. In the lymphocele group (n=50), 13 patients were diagnosed with complicated lymphocele. The BMI and number of resected pelvic lymph nodes were found to be higher in the lymphocele than in the non-lymphocele group (23.94±3.38 vs. 22.52±3.00, p=0.00 and 26.80±14.82 vs. 22.96±10.18, p=0.03, respectively), and showed statistical significance. The occurrence of lymphoceles was lower without postoperative radiotherapy (p=0.01).
Conclusion
Among the 264 patients, a total of 50 patients (18%) developed lymphoceles. The BMI and number of resected lymph nodes were higher in the lymphocele group, and the use of postoperative radiotherapy was associated with a higher risk of lymphoceles. Thirteen of the 50 patients that developed lymphoceles (n=50) required treatment for lymphocele-related complications.
Pelvic lymphadenectomy is commonly used to assess lymph node status, evaluate the stage and for treating gynecologic malignancies, and is often accompanied by complications, such as hemorrhage or hematoma, postoperative ileus and lymphocele. Of these complications, lymphocele is one of the most common postoperative complications (1).
The definition of a lymphocele is a lymph-filled extraperitoneal space, with no epithelial lining. Most lymphoceles occur within 3~8 weeks, but may occur 1 year after surgery. Previous authors have reported a high incidence of lymphocele after a pelvic lymphadenectomy. Mori, for example, reported an average incidence of 48.5% (1). However, more recent series have reported much lower incidences. Ilancheran et al. noted an incidence of 25.3% in clinically diagnosable lymphocele with cervical cancer (2), Conte et al. noted an incidence of 22% in cervical cancer (3), Logmans et al. an incidence of 20~32% in gynecologic cancer (4) and Panici et al. an incidence of 10~25% in gynecologic cancer (5). The reported incidences of lymphoceles following pelvic lymphadenectomy vary, which is probably related to the method of identification used. Whereas, clinical examination alone may be insufficient to detect a small lymphocele, but prospective scans of the pelvis by ultrasound, CT (computed tomography) scan or MRI (magnetic resonance imaging) offer more objective methods.
Several factors may contribute to this varied incidence. Lymphocele developmental factors are thought to include; the extent of lymphadenectomy, the number of lymph nodes removed, no ligation of lymph vessels, pre/postoperative radiation therapy and the presence of metastases to the lymph nodes (6). Other factors that may influence its development include; the use of retroperitoneal suction drainage (7) and low-dose heparin thromboembolic prophylaxis (8).
Although many lymphoceles are incidental findings having little clinical significance, some can cause problems. Whereas, small lymphoceles are often asymptomatic, infected or large collections may cause fever, abdominal pain, tenesmus, urinary frequency, hydronephrosis, leg edema and/or deep venous thrombosis (9~12). The symptomatology varies depending on the location of the lymphoceles and the effects of pressure on the surrounding structures.
The treatment of small, uninfected lymphoceles is generally conservative; on the other hand, large collections are not as likely to be resorbed spontaneously; thus, require more aggressive approaches, including fine-needle aspiration, sclerotherapy, catheter drainage and/or surgical marsupialization (13).
In these studies, our objective was to evaluate the incidence of lymphocele to identify the risk factors of lymphocele development after a pelvic lymphadenectomy and to investigate their management.
This retrospective study was carried out on 264 gynecologic cancer patients who received pelvic lymphadenectomy and paraaortic lymphadenectomy, at the Severance Hospital, Yonsei University of Medical Center, Seoul, Korea, between March 1999 and February 2003. 105 patients had cervical cancer, 115 ovarian cancer, 40 endometrial cancer and 4 had other malignancies. Patients were classified into two groups; the lymphocele (n=50) and the non-lymphocele groups (n=214), as confirmed by ultrasonography, CT scan and MRI. These groups were compared with respect to possible risk factors, i.e., type and stage of cancer, BMI (body mass index), preoperative Hb, the use of pre/postoperative chemotherapy or radiotherapy, the number of resected pelvic lymph nodes and the volume of postoperative drainage from a Hemovac® pelvic drain. The case records of the 50 patients with lymphocele were scrutinized for possible contributory factors, complications and management during postoperative follow-up examinations every 3~4 weeks.
Almost all primary surgeries were carried out by one surgeon. These involved unilateral or bilateral pelvic lymphadenectomies. For these patients, the lymphadenectomy was associated with hysterectomies, salpingooophorectomies, omentectomies and multiple peritoneal biopsies, with or without bowel resection, depending on the tumor intraabdominal spread. The surgical technique has been previously described by Benedetti-Panici et al, as follows (14). Nodes were dissected from vessels of the adventitia by blunt and sharp dissection. Vessel mobilization was performed with small waves and rubber tape in order to remove all the lymphofatty tissue surrounding the aorta, cava and pelvic vessels. Careful hemostasis was performed. No special effort was made to tie off all lymphatic channels, although surgiclips were used liberally during the procedure. The para-aortic lymphadenectomy included the removal of the lymphofatty tissue around and between the aorta and the cava, was performed. The pelvic lymphadenectomy included removal of the common iliac, external iliac and obturator node groups. For the purposes of this study, the surgical technique was modified as follows: the pelvic peritoneum and the peritoneum along the paracolic gutters were sutured with continuous 2-0 catgut (Chromic, Ethicon Ltd.), after reapproximation of the peritoneum. The fascia was closed with a running 0 polydioxanone (PDS II, Ethicon Ltd.) or 2-0 polyglactin 910 suture (Vicryl; Ethicon Ltd., Somerville, NJ) in all patients. Patients with a sutured peritoneum were drained with a Hemovac® drain (Sewoon-medical), with or without low-pressure suction apparatus. The volume of fluid from each drain was measured daily. The drains were removed when the fluid drainage was <50 ml/day. No patient was discharged with a drain in place. A pathological examination was performed on lymphatic tissue and lymph nodes were counted and submitted for histological analysis to determine metastatic involvement. All patients received intraoperative antibiotic prophylaxis, which was continuing postoperatively until complete ambulation had been restored. Postoperative follow-up included assessments of febrile morbidity, the duration and amount of fluid drainage, and complications. The clinical detection of lymphoceles was documented at subsequent review visits. Follow-up scans were not performed routinely.
Ultrasonography revealed a thin-walled pelvic "cyst" in 50 of the 264 patients (18%) (Fig. 1A). Lymphoceles were visualized as well-circumscribed oval structures, and sometimes contained multiple septa. Cystic structures arising from the pelvic wall, separate from the ovaries (when preserved), were assumed to be lymphoceles. CT findings of a smooth and thin-walled cavity filled with a water-equivalent fluid, which was sharply demarcated from its surroundings, and showing no signs of infiltration, were interpreted as lymphoceles (Fig. 1B). In MRI, because of their protein contents, the lymphoceles had high signal intensities on T2-W1. The configuration and position of lymphoceles, together with their signal characteristics, facilitated their recognition by imaging studies, particularly with a previous history of lymph node dissection (Fig. 1C). By physical examination, clinical lymphoceles were detectable in 11 of the 50 patients. By CT scan and MRI, lymphoceles were visualized in 41 and 6 of the 50 patients, respectively. The time taken for detection of lymphoceles varied from 3 weeks to 6 months postoperatively, with a mean of 1.8 months. In 38 cases, the occurrence was unilateral (Right=15, Left=23), while in the other 12 cases this was bilateral.
The incidences of lymphocele, according to primary cancer type, were as follows (Table 1); Pelvic lymphoceles were diagnosed in 18% (50 of 264) of the total study population, in 14% (15 of 105) of cervical cancer patients, 19% (22 of 115) of ovarian cancer patients and in 27% (11 of 40) of endometrial cancer patients.
Each group was compared with respect to possible risk factors of lymphocele development (Table 2). BMI was found to be significantly higher in the lymphocele (23.94±3.38 vs. 22.52±3.00, p=0.00) than the non-lymphocele group and the number of resected pelvic lymph nodes was significantly higher in the lymphocele group (26.80±14.82 vs. 22.96±10.18, p=0.03). Of the 264 cases, only 32 patients were found to have positive nodes at surgery, 12 in the lymphocele and 20 in the non-lymphocele group (p=0.04). The incidence of patients with lymph node metastases was 12% (32 of 264). No significant differences were found with respect to age, preoperative Hb, duration until lymphocele occurrence or amount of postoperative pelvic drainage. Patients with drains had a median fluid loss of 140.2±80.8 ml/day in the lymphocele and 148.7±91.8 ml/day in the non-lymphocele group, with median drainage durations of 6.3 and 7.1 days, respectively.
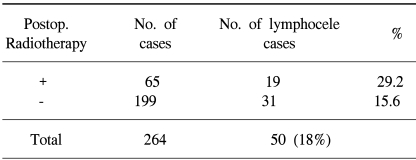
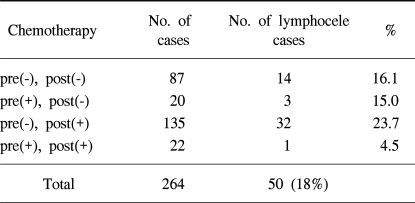
84 patients received postoperative radiotherapy, but no patient received preoperative radiotherapy, whereas 46 received preoperative chemotherapy and 190 postoperative chemotherapy and the occurrence of lymphocele was lower for those without postoperative radiotherapy (p=0.01) (Table 3). No significant differences were observed with respect to pre/postoperative chemotherapies (p=0.10) (Table 4).
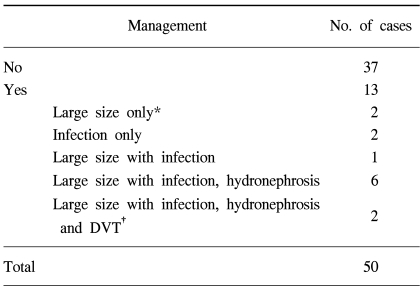
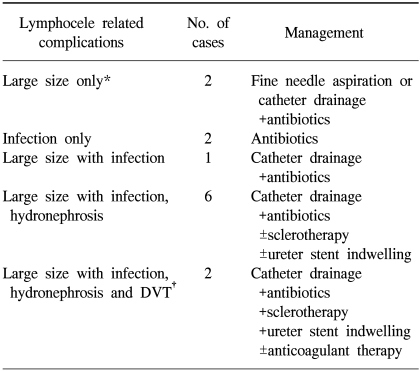
In our series, 13 of the 50 patients that developed lymphocele needed treatment for lymphocele related complications (Table 5). In the lymphocele group (n=50), eleven of the 13 complained of pelvic pain or leg swelling, and all 13 suffered from mild fever. Some patients had more than one symptom; 2 developed a large (>6 cm) lymphocele, 2 an infected lymphocele, 1 a large infected lymphocele, 6 an infected lymphocele, with hydronephrosis, and a further 2 an infected lymphocele, with hydronephrosis and deep vein thrombosis. Of the 50 lymphocele patients, 11 (22%) had a lymphocele <3 cm in diameter, 26 (52%) 3~6 cm and 13 (26%) were >6 cm in diameter. All lymphoceles with a diameter of <6 cm were asymptomatic, whereas all but 13 lymphoceles with a diameter exceeding 6 cm were symptomatic.
The remaining 37 patients were not treated. Regression of the lymphoceles occurred over several months in 25 of the 37 patients, but persistent lymphoceles were noted in the other 12.
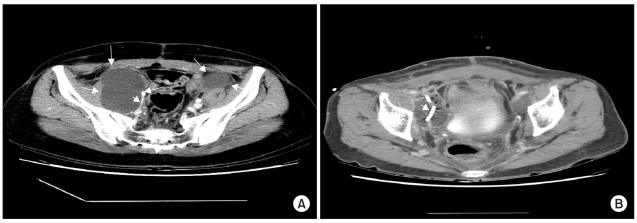
Of the 13 complicated lymphocele patients, none required a laparotomy and marsupialization (Table 6), but all received antibiotic therapy. Two patients were adequately treated by antibiotics alone. Only one patient underwent fine-needle aspiration without image-guidance. Catheter drainage under sonographic guidance was performed in ten patients (Fig. 2). One patient with a single large (>6 cm) lymphocele and another with a large infected lymphocele required catheter drainage. Of the 6 patients with a large infected lymphocele, with hydronephrosis, catheter drainage, with sclerotherapy and ureter stent indwelling, was performed in 2 cases, catheter drainage with sclerotherapy in 1, catheter drainage with ureter stent indwelling in 2 and catheter drainage alone in a further case. Of the two patients with a large infected lymphocele, with hydronephrosis and deep vein thrombosis, one received catheter drainage with sclerotherapy and ureter stent indwelling and in the other, additional anticoagulant therapy was needed. No patient experienced sepsis, bowel perforation or neurovascular injury. Catheter drainage allowed complete clinical and radiological remission in all cases (Fig. 3A,B), which was tolerated and caused no side effects at either the short- or long-term follow-ups.
A lymphocele is a retroperitoneal collection of lymphatic fluid contained within a sac, which commonly arise secondary to lymphatic channel injury (1,2,15). A lymphocele occurs after renal transplantation and surgery due to prostatic, gynecologic malignancies, a vascular disease (15,16) or lymphatic vessel injury after a pelvic lymphadenectomy. In the present study, all lymphoceles were located in the pelvis, thus confirming that the rarity of aortic lymphoceles (17,18). Moreover, the incidence of lymphoceles was found to be similar for pelvic lymphadenectomies, with or without a para-aortic lymphadenectomy. These findings suggest that lymphoceles are a specific complication of pelvic lymphadenectomies (2,9). Following the removal of lymph nodes and adipose tissue, a space or potential space is formed, which is bounded by the denuded musculature, both laterally and posteriorly, and by the peritoneum medially (9). Although this space is spontaneously obliterated in the majority of patients, lymph accumulation occurs in others. The resulting mass varies in size, and may apply variable pressures on the ureter, bladder, rectosigmoid and blood vessels. In the majority of patients these effects may be minimal (i.e., asymptomatic), and are rarely severe enough to interfere with organ function.
Lymphoceles have occurred in approximately 10 to 25% of previous studies, and in most are asymptomatic and disappear spontaneously (2,5,19). However, in 5 to 10% of patients, the lymphoceles become symptomatic, manifesting fever, pain, constipation, prolonged ileus, urinary frequency, obstructive uropathy, leg edema, erythema of overlying skin or deep vein thrombosis (11,20). In our series, the incidence rate of symptomatic lymphocele was 18% (Table 1), which was comparable with the rates reported by Conte et al. (3) and Petru et al. (17), although is rather high compared with figures published more recently (14,21). However, our series included both symptomatic and progressive asymptomatic cases, which undoubtedly influenced the observed incidence of lymphoceles.
Lymphoceles can be detected by clinical examination, ultrasonography, intravenous urography, CT scan or MRI. The differential diagnosis includes; urinoma, seroma, hematoma, abscess and a cystic neoplasm. Lymphoceles typically become detectable within 3~8 weeks postoperatively (3,20). Those that occur later than 1 year after surgery should be suspected of harboring a recurrent disease, although in our experience lymphocele development has not occurred more than 1 year after surgery. Most of the lymphoceles in the present series regressed spontaneously; 37 of the 50 patients that underwent a CT scan showed resolution or a decreased size.
The formation of lymphoceles may be promoted by several factors: the extent of lymphadenectomy (9,10,12), the number of lymph nodes removed (22,23); no ligation of lymph vessels (24); the presence of metastases to the lymph nodes (9); pre/postoperative radiation therapy (8,11) and the prophylactic use of anticoagulants (11). However, none of these potential factors have been proven to be significant. In our series, the lymphocele and non-lymphocele groups were significantly different in terms of the number of resected lymph nodes, BMI and the use of postoperative radiotherapy (Table 2, 3). Efforts to prevent lymphocele formation have only been partially successful. Such methods include; strict indications and well-defined dissection boundaries, retroperitoneal suction drainage (7,16), and the prophylactic use of antibiotics (11).
The treatment of lymphocele should be individualized. If they are asymptomatic, they should be monitored for spontaneous regression. In the majority of cases this will lead to successful resolution, as in the present series. However, regression may be a slow process; occurring over several months. The management of lymphoceles has involved several strategies, including percutaneous needle aspiration, percutaneous catheter drainage, sclerotherapy and open drainage by laparotomy, as well as laparoscopy. Asymptomatic lymphoceles <100 ml can be followed clinically, and will usually resolve spontaneously. Lymphoceles that persist for >6 months or become symptomatic can be reduced by ultrasonography or CT scan-guided percutaneous needle aspiration. The decision to puncture a lymphocele depends mainly on its location. Aspirated fluids should be sent for chemistry, cytology and culture, in order to establish a diagnosis and rule out malignancy or infection. Large or recurrent lymphoceles may be managed by sonography or CT scan-guided placement of a percutaneous catheter, with drainage. The catheter is left within the lymphocele until drainage has diminished to 50 ml per 24h and lymphocele resolution has been demonstrated radiographically. The morbidity associated with this procedure is lower than those of invasive surgical procedures, with the successful resolution of lymphoceles using this method having been reported in between 79 and 82% of cases (19). Ultrasonography or CT scan-guided percutaneous aspiration, followed with sclerotherapy, is another treatment modality. Some studies have described lymphocele treatment by percutaneous catheter drainage and sclerotherapy with 95% absolute ethanol, 105 povidone iodine solution, tetracycline, sodium tetradecyl sulfate, or doxycycline. Lymphoceles rarely require a laparotomy and intraperitoneal marsupialization. For infected lymphoceles, antibiotics alone are often sufficient. However, the identities of the common pathogens that cause a lymphocele infection, and the route of the infection, remain unclear. However, infections are largely of theoretical concern, as they may occur in the early postoperative period during the re-epithelization process and related to the accumulation of fluids (25). In our series, 13 of the 50 patients with lymphoceles needed treatment due to lymphocele related complications (Table 5, 6); the remaining 37 were not treated. No patient required a laparotomy and marsupialization. All 13 treated patients were administered antibiotics. Two patients responded adequately to antibiotic treatment alone. Only one patient underwent fine-needle aspiration without image-guidance. Catheter drainage under sonographic guidance was performed in ten patients. No patient experienced sepsis, bowel perforation or neurovascular injury.
Serial radiological examinations in the patients that underwent pelvic and para-aortic lymphadenectomies allowed for the early detection and treatment of symptomatic and progressive asymptomatic collections, which possibly reduced the duration of therapy and the risk of further complications. However, most lymphocele patients are asymptomatic, and rarely needed treatment. Therefore, it is our belief that close clinical examinations are required in patients having undergone a pelvic lymphadenectomy.
In the present study, of the 264 patients, 50 (18%) developed lymphoceles, and it was indicated that the number of resected lymph nodes, the BMI and use of postoperative radiotherapy should be viewed as risk factors for lymphocele development. In the lymphocele group (n=50), 13 patients needed treatment due to lymphocele-associated complications.
References
1. Mori N. Clinical and experimental studies on the so-called lymphocyst which develops after radical hysterectomy in cancer of the uterine cervix. J Jpn Obstet Gynecol Soc. 1955; 2:178–203. PMID: 13286539.
2. Ilancheran A, Monaghan JM. Pelvic lymphocyst--a 10-year experience. Gynecol Oncol. 1988; 29:333–336. PMID: 3345953.

3. Conte M, Panici PB, Guariglia L, Scambia G, Greggi S, Mancuso S. Pelvic lymphocele following radical para-aortic and pelvic lymphadenectomy for cervical carcinoma: incidence rate and percutaneous management. Obstet Gynecol. 1990; 76:268–271. PMID: 2196500.
4. Logmans A, Kruyt RH, de Bruin HG, Cox PH, Pillay M, Trimbos JB. Lymphedema and lymphocysts following lymphadenectomy may be prevented by omentoplasty: A pilot study. Gynecol Oncol. 1999; 75:323–327. PMID: 10600283.

5. Benedetti-Panici P, Maneschi F, Cutillo G. Pelvic and aortic lymphadenectomy. Surg Clin North Am. 2001; 81:841–858. PMID: 11551129.

6. Shingleton HM, Orr HW. Cancer of the cervix: Diagnosis and Treatment in Current reviews in obstetrics and gynecology. 1993. Vol. 5. Edinburgh: Churchill Livingstone.
7. Symmonds RE, Pratt JH. Prevention of fistulas and lymphocysts in radical hysterectomy. Preliminary report of a new technic. Obstet Gynecol. 1961; 17:57–64. PMID: 13774382.
8. Catalona WJ, Kadmon D, Crane DB. Effect of mini-dose heparin on lymphocele formation following extraperitoneal pelvic lymphadenectomy. J Urol. 1980; 123:890–892. PMID: 7382008.

9. Rutledge F, Dodd GD Jr, Kasilag FB Jr. Lymphocysts; a complication of radical pelvic surgery. Am J Obstet Gynecol. 1959; 77:1165–1175. PMID: 13649788.

10. Pennehouat G, Mosseri V, Durand JC, Hamelin JP, Asselain B, Pilleron JP, et al. Lymphoceles and peritonization following lymphadenectomy for cancer of the uterus. J Gynecol Obstet Biol Reprod (Paris). 1988; 17:373–378. PMID: 3294282.
11. Clarke-Pearson DL, Jelovsek FR, Creasman WT. Thromboembolism complicating surgery for cervical and uterine malignancy: incidence, risk factors, and prophylaxis. Obstet Gynecol. 1983; 61:87–94. PMID: 6823353.
12. Orr JW Jr, Barter JF, Kilgore LC, Soong SJ, Shingleton HM, Hatch KD. Closed suction pelvic drainage after radical pelvic surgical procedures. Am J Obstet Gynecol. 1986; 155:867–871. PMID: 3766643.

13. White M, Mueller PR, Ferrucci JT Jr, Butch RJ, Simeone JF, Neff CC, et al. Percutaneous drainage of postoperative abdominal and pelvic lymphoceles. AJR Am J Roentgenol. 1985; 145:1065–1069. PMID: 3901705.

14. Benedetti-Panici P, Maneschi F, Scambia G, Mancuso S. A new transabdominal approach to the left retroperitoneum for systematic removal of lymph nodes left of the aorta in gynecologic malignancies. Obstet Gynecol. 1994; 83:1060–1064. PMID: 8190424.

15. Metcalf KS, Peel KR. Lymphocele. Ann R Coll Surg Engl. 1993; 75:387–392. PMID: 8285540.
16. Blebea J, Choudry R. Thigh isosulfan blue injection in the treatment of postoperative lymphatic complications. J Vasc Surg. 1999; 30:350–354. PMID: 10436456.

17. Petru E, Tamussino K, Lahousen M, Winter R, Pickel H, Haas J. Pelvic and paraaortic lymphocysts after radical surgery because of cervical and ovarian cancer. Am J Obstet Gynecol. 1989; 161:937–941. PMID: 2801842.

18. Livingston WD, Confer DJ, Smith RB. Large lymphoceles resulting from retroperitoneal lymphadenectomy. J Urol. 1980; 124:543–546. PMID: 7420600.

19. Spiegel RA, Hessel SJ, Katz ER, Levy JM. Pelvic fullness, urgency and frequency following lymphadenectomy. Pelvic lymphocele. West J Med. 1987; 147:357–358. PMID: 3673073.
20. Terada KY, Roberts JA. Lymphoceles following second-look laparotomy for ovarian cancer. Gynecol Oncol. 1988; 29:382–384. PMID: 3345957.

21. Benedet JL, Turko M, Boyes DA, Nickerson KG, Bienkowska BT. Radical hysterectomy in the treatment of cervical cancer. Am J Obstet Gynecol. 1980; 137:254–262. PMID: 7377245.

22. Nelson JH Jr, HUston JW. Lymphocyst formation following pelvic lymphadenectomy. Am J Obstet Gynecol. 1959; 78:1298–1300. PMID: 14426460.

23. de Roo T. The value of lymphography in lymphedema. Surg Gynecol Obstet. 1967; 124:755–765. PMID: 6019288.
24. Ferguson JH, Maclure JG. Lymphocele following lymphadenectomy. Am J Obstet Gynecol. 1961; 82:783–792. PMID: 13892338.

25. Fung YM, Wong WS. Malignant lymphocyst after Wertheim's operation. Gynecol Oncol. 1992; 44:288–290. PMID: 1541444.

Fig. 1
Radiological examination showing lymphocele. (A) Ultrasonography revealed a thin-walled pelvic "cyst" (about 7×3 cm2 sized). Lymphoceles were visualized as well-circumscribed oval structures. (B) CT findings of a smooth and thin-walled cavity (arrow) filled with a water-equivalent fluid, which was sharply demarcated from its surroundings, and showing no signs of infiltration were interpreted as lymphoceles. After ultrasonography-guided percutaneous catheter drainage, a catheter (arrow head) was inserted in the lymphocele cavity. (C) MRI revealed an about 7×3 cm2 sized cyst, with low signal intensity on T1-W1.
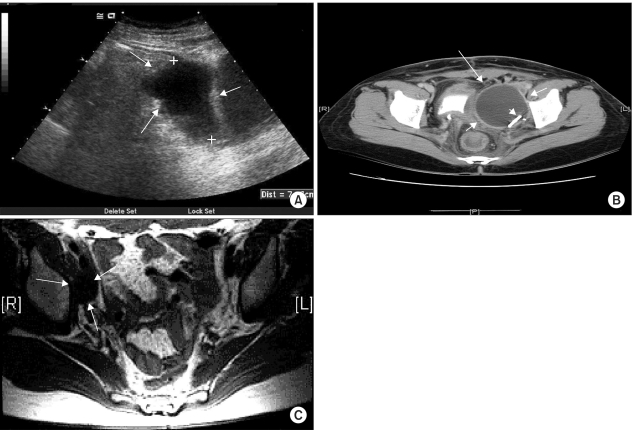
Fig. 2
Fluoroscopy-guided percutaneous catheter drainage (after pelvic sonography). Arrow head; catheter
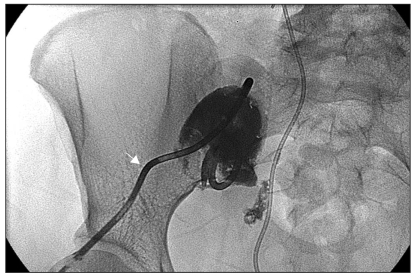
Fig. 3
Percutaneous catheter drainage. (A) Large (about 11×7 cm2 sized) lymphocele were managed using sonography-guided placement of a percutaneous catheter, with drainage. The catheter (arrow head) is at the left, within the lymphocele. (B) Catheter drainage allowed complete radiological remission in this case. Arrow head; catheter.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download