1. Cameliet P, Jain RK. Angiogenesis in cancer and other disease. Nature. 2000; 407:249–257. PMID:
11001068.
2. Mazure NM, Brahimi-Horn MC, Berta MA, Benizri E, Bilton RL, Dayan F, et al. HIF-1: master and commander of the hypoxic world. A pharmacological approach to its regulation by siRNAs. Biochem Pharmacol. 2004; 68:971–980. PMID:
15313390.
3. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003; 3:721–732. PMID:
13130303.

4. Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol. 1999; 22:172–178. PMID:
1848205.

5. Wang GL, Jiang BH, Semenza GL. Effect of protein kinase and phosphatase inhibitors on expression of HIF-1. Biochem Biophys Res Commun. 1995; 216:669–675. PMID:
7488163.
6. Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of HIF-1α. J Biol Chem. 1996; 271:17771–17778. PMID:
8663540.
7. Ruas JL, Poellinger L, Pereira T. Functional analysis of hypoxia-inducible factor-1 alpha-mediated transactivation. Identification of amino acid residues critical for transcriptional activation and/or interaction with CREB-binding protein. J Biol Chem. 2002; 277:38723–38730. PMID:
12133832.
8. Tian H, Mcknight SL, Russel DW. Endothelial PAS domain protein 1, a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997; 11:72–82. PMID:
9000051.
9. Gothie E, Richard DE, Berra E, Pages G, Pouyssegur J. Identification of alternative spliced variants of human hypoxic-inducible factor-1alpha. J Biol Chem. 2000; 275:6922–6927. PMID:
10702253.
10. Chun YS, Choi EJ, Yeo EJ, Lee JH, Kim MS, Park JW. A new HIF-1 alpha variant induced by zinc ion suppresses HIF-1-mediated hypoxic responses. J Cell Sci. 2001; 114:4051–4061. PMID:
11739637.

11. Chun YS, Choi EJ, Kim TY, Kim MS, Park JW. A dominant-negative isoform lacking exon 11 and 12 of the human hypoxic-inducible factor-1α gene. Biochem J. 2002; 362:71–79. PMID:
11829741.
12. Chun YS, Lee KH, Choi EJ, Bae SY, Yeo EJ, Huang LE, et al. Phorbol ester stimulates the nonhypoxic induction of a novel hypoxia-inducible factor 1α isoform: implications for tumor promotion. Cancer Res. 2003; 63:8700–8707. PMID:
14695184.
13. Lee KH, Park JW, Chun YS. Non-hypoxic transcriptional activation of the aryl hydrocarbon receptor nuclear translocator in concert with a novel hypoxia-inducible factor-1alpha isoform. Nucleic Acids Res. 2004; 32:5499–5511. PMID:
15479785.

14. Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001; 292:464–468. PMID:
11292862.
15. Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001; 20:5197–5206. PMID:
11566883.
16. Maxwell P, Weisner M, Chang GW, Clifford S, Vaux E, Pugh C, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999; 399:271–275. PMID:
10353251.

17. Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia inducible factor-1 alpha by the von Hipple-Lindau tumor suppressor protein. EMBO J. 2000; 19:4298–4309. PMID:
10944113.
18. Maynard MA, Qi H, Chung J, Lee EH, Kondo Y, Hara S, et al. Multiple splice variants of the human HIF-3alpha locus are targets of the VHL E3 ubiquitin ligase complex. J Biol Chem. 2003; 278:11032–11040. PMID:
12538644.
19. Makino Y, Kanopka A, Wilson WJ, Tanaka H, Poellinger L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3α locus. J Biol Chem. 2002; 277:32405–32408. PMID:
12119283.

20. Heidbreder M, Frohlich F, Johren O, Dendorfer A, Qadri F, Dominiak P. Hypoxia rapidly activates HIF-3α mRNA expression. FASEB J. 2003; 17:1541–1543. PMID:
12824304.

21. Rosenberger C, Mandriota S, Jurgensen JS, Wiesner MS, Horstrup JH, Frei U, et al. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002; 13:1974–1976. PMID:
12089396.
22. Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD. The contribution of VHL substrate binding and HIF-1alphaz to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002; 1:247–255. PMID:
12086861.
23. Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG. Inhibition of HIF is necessary for tumor suppression by the von Hippel Lindau protein. Cancer Cell. 2002; 1:237–246. PMID:
12086860.
24. Blancher C, Moore JW, Talks KL, Houlbrook S, Harris AL. Relationship of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Res. 2002; 60:7106–7113. PMID:
11156418.
25. Berta M, Mazure N, Hattab M, Pouyssegur J, Brahimi-Horn MC. Hypoxia-inducible factor-1a is modified by covalent attachment to SUMO. Keystone Symposia Conference: Biology of Hypoxia: The Role of Oxygen Sensing in Development, Normal Function and Disease. 2004.
26. An FQ, Matsuda M, Fujii H, Matsumoto Y. Expression of vascular endothelial growth factor in surgical specimens of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2000; 126:153–160. PMID:
10741909.

27. Berra E, Pages G, Pouyssegur J. MAP kinases and hypoxia in the control of VEGF expression. Cancer Metastasis Rev. 2000; 19:139–145. PMID:
11191053.
28. Josko J, Gwozdz B, Jedrzejowska-Szypulka H, Hendryk S. Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med Sci Monit. 2000; 6:1047–1052. PMID:
11208453.
29. Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001; 49:507–521. PMID:
11166264.

30. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999; 13:9–22. PMID:
9872925.

31. Harris AL. von Hippel-Lindau syndrome: target for anti-vascular endothelial growth factor (VEGF) receptor therapy. Oncologist. 2000; 5(Suppl.1):32–36. PMID:
10804089.

32. Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med. 1995; 182:1683–1693. PMID:
7500013.

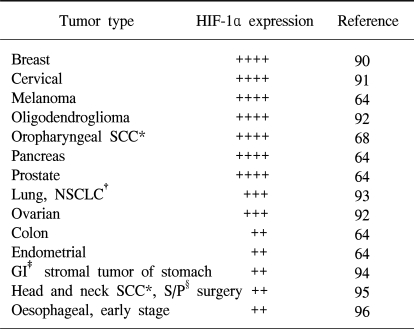
33. Palmer LA, Semenza GL, Stoler MH, Johns RA. Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol. 1998; 274:L212–L219. PMID:
9486205.
34. Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, et al. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997; 272:5375–5381. PMID:
9038135.

35. Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998; 245:894–899. PMID:
9588211.

36. Nguyen SV, Claycomb WC. Hypoxia regulates the expression of the adrenomedullin and HIF-1 genes in cultured HL-1 cardiomyocytes. Biochem Biophys Res Commun. 1999; 265:382–386. PMID:
10558876.

37. Eckhart AD, Yang N, Xin X, Faber JE. Characterization of the alpha1B-adrenergic receptor gene promoter region and hypoxia regulatory elements in vascular smooth muscle. Proc Natl Acad Sci USA. 1997; 94:9487–9492. PMID:
9256509.
38. Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002; 16:1151–1162. PMID:
12153983.

39. Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003; 63:1138–1143. PMID:
12615733.
40. Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999; 59:3915–3918. PMID:
10463582.
41. Chen EY, Mazure NM, Cooper JA, Giaccia AJ. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/Akt pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 2001; 61:2429–2433. PMID:
11289110.
42. Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000; 14:391–396. PMID:
10691731.

43. Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002; 2:38–47. PMID:
11902584.
44. Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999; 24:68–72. PMID:
10098401.

45. Seagroves TN, Ryan HE, Lu H, Wouters BG, Knapp M, Thibault P, et al. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol Cell Biol. 2001; 21:3436–3444. PMID:
11313469.

46. Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol. 2000; 203:1253–1263. PMID:
10729275.

47. Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001; 276:9519–9525. PMID:
11120745.
48. Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000; 60:7075–7083. PMID:
11156414.
49. Mazurek S, Boschek CB, Eigenbrodt E. The role of phosphometabolites in cell proliferation, energy metabolism, and tumor therapy. J Bioenerg Biomembr. 1997; 29:315–330. PMID:
9387092.
50. Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of HIF depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996; 271:32253–32259. PMID:
8943284.
51. Kallio PJ, Pongratz I, Gradin K, Mcguire J, Poellinger L. Activation of hypoxia-inducible factor 1alpha: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci USA. 1997; 94:5667–5672. PMID:
9159130.
52. Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, Pugh CW, et al. Structural basis for the recognition of hydroxyproline in HIF-1α by pVHL. Nature. 2002; 417:975–978. PMID:
12050673.

53. Min JH, Yang H, Ivan M, Gertler F, Kaelin WG Jr, Pavletich NP. Structure of an HIF-1α-pVHL complex: Hydroxyproline recognition in signalling. Science. 2002; 296:1886–1889. PMID:
12004076.
54. Huang J, Zhao Q, Mooney SM, Lee FS. Sequence determinants in hypoxia inducible factor-1α for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J Biol Chem. 2002; 277:39792–39800. PMID:
12181324.
55. Metzen E, Berchner-Pfannschmidt U, Stengel P, Marxsen JH, Stolze I. Intracellular localization of human HIF-1α hydroxylases: Implications for oxygen sensing. J Cell Sci. 2003; 116:1319–1326. PMID:
12615973.
56. Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001; 107:43–54. PMID:
11595184.

57. Jewell UR, Kveitikova I, Scheid A, Bauer C, Wenger RH, Gassmann M. Induction of Hif-1alpha in response to hypoxia is instantaneous. FASEB J. 2001; 15:1312–1314. PMID:
11344124.
58. Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002; 111:709–720. PMID:
12464182.
59. Tribioli C, Mancini M, Plassart E, Bione S, Rivella S, Sala C, et al. Isolation of new genes in distal Xq28: transcriptional map and identification of a human holologue of the ARD1 N-acetyl transferase of Saccharomyces cerevisiae. Human Mol Genet. 1994; 3:1061–1067. PMID:
7981673.
60. Ingram AK, Cross GA, Horn D. Genetic manipulation indicates that ARD1 is an essential N-acetyltransferase in Trypansoma brucei. Mol Biochem Parasitol. 2000; 111:309–317. PMID:
11163439.
61. Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000; 19:4298–4309. PMID:
10944113.
62. Bae SH, Jeong JW, Park JA, Kim SH, Bae MK, Choi SJ, et al. Sumoylation increases HIF-1alpha stability and its transcriptional activity. Biochem Biophys Res Commun. 2004; 324:394–400. PMID:
15465032.
63. Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000; 157:411–421. PMID:
10934146.
64. Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999; 59:5830–5835. PMID:
10582706.
65. Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997; 90:3322–3331. PMID:
9345014.

66. Lando D, Gorman JJ, Whitelaw ML, Peet DJ. Oxygen-dependent regulation of hypoxia-inducible factors by prolyl and asparaginyl hydroxylation. Eur J Biochem. 2003; 270:781–790. PMID:
12603311.

67. Unruh A, Ressel A, Mohamed HG, Johnson RS, Nadrowitz R, Richter E, et al. The hypoxia-inducible factor-1 alpha is a negative factor for tumor therapy. Oncogene. 2003; 22:3213–3220. PMID:
12761491.
68. Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, et al. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001; 61:2911–2916. PMID:
11306467.
69. Sun X, Kanwar JR, Leung E, Lehnert K, Wang D, Krissansen GW. Gene transfer of antisense hypoxia inducible factor-1 alpha enhances the therapeutic efficacy of cancer immunotherapy. Gene Ther. 2001; 8:638–645. PMID:
11320410.
70. Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med. 2000; 6:1335–1340. PMID:
11100117.

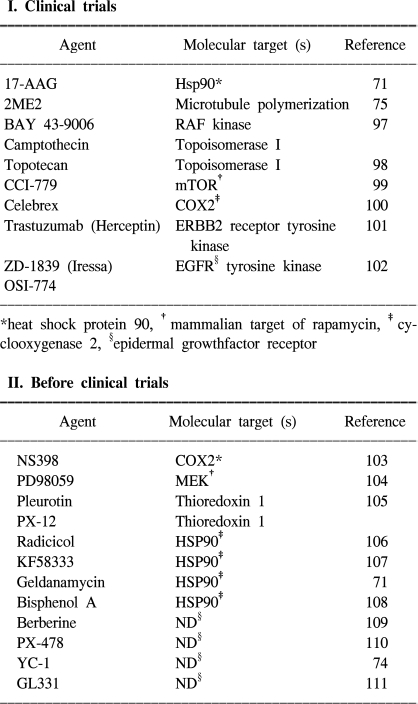
71. Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 2002; 277:29936–29944. PMID:
12052835.
72. Mabjeesh NJ, Post DE, Willard MT, Kaur B, Van Meir EG, Simons JW, et al. Geldanamycin induces degradation of hypoxia-inducible factor 1alpha protein via the proteasome pathway in prostate cancer cells. Cancer Res. 2002; 62:2478–2482. PMID:
11980636.
73. Zagzag D, Nomura M, Friedlander DR, Blanco CY, Gagner JP, Nomura N, et al. Geldanamycin inhibits migration of glioma cells in vitro: a potential role for hypoxia-inducible factor (HIF-1alpha) in glioma cell invasion. J Cell Physiol. 2003; 196:394–402. PMID:
12811834.
74. Yeo EJ, Chun YS, Cho YS, Kim J, Lee JC, Kim MS, et al. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst. 2003; 95:516–525. PMID:
12671019.

75. Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003; 3:363–375. PMID:
12726862.

76. Dachs GU, Patterson AV, Firth JD, Ratcliffe PJ, Townsend KM, Stratford IJ, et al. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997; 3:515–520. PMID:
9142119.

77. Lemmon MJ, van Zijl P, Fox ME, Mauchline ML, Giaccia AJ, Minton NP, et al. Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment. Gene Ther. 1997; 4:791–796. PMID:
9338007.

78. Sutter CH, Laughner E, Semenza GL. Hypoxia-inducible factor 1alpha protein expression is controlled by oxygen-regulated ubiquitination that is disrupted by deletions and missense mutations. Proc Natl Acad Sci USA. 2000; 97:4748–4753. PMID:
10758161.

79. Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, et al. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000; 275:25733–25741. PMID:
10823831.
80. Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci USA. 2000; 97:10430–10435. PMID:
10973499.

81. Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997; 272:22642–22647. PMID:
9278421.
82. Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000; 14:34–44. PMID:
10640274.
83. An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998; 392:405–408. PMID:
9537326.
84. Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, et al. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999; 18:1905–1914. PMID:
10202154.

85. Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, et al. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA. 1996; 93:12969–12973. PMID:
8917528.

86. Carrero P, Okamoto K, Coumailleau P, O'Brien S, Tanaka H, Poellinger L. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1alpha. Mol Cell Biol. 2000; 20:402–415. PMID:
10594042.
87. Minet E, Mottet D, Michel G, Roland I, Raes M, Remacle J, et al. Hypoxia-induced activation of HIF-1: role of HIF-1alpha-Hsp90 interaction. FEBS Lett. 1999; 460:251–256. PMID:
10544245.
88. Bae MK, Ahn MY, Jeong JW, Bae MH, Lee YM, Bae SK, et al. Jab1 interacts directly with HIF-1alpha and regulates its stability. J Biol Chem. 2002; 277:9–12. PMID:
11707426.
89. Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000; 88:1474–1480. PMID:
10749844.

90. Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, et al. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res. 2002; 8:1831–1837. PMID:
12060624.
91. Burri P, Djonov V, Aebersold DM, Lindel K, Studer U, Altermatt HJ, et al. Significant correlation of hypoxia-inducible factor-1alpha with treatment outcome in cervical cancer treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003; 56:494–501. PMID:
12738326.
92. Birner P, Gatterbauer B, Oberhuber G, Schindl M, Rossler K, Prodinger A, et al. Expression of hypoxia-inducible factor-1 alpha in oligodendrogliomas: its impact on prognosis and on neoangiogenesis. Cancer. 2001; 92:165–171. PMID:
11443623.
93. Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001; 85:881–890. PMID:
11556841.
94. Takahashi R, Tanaka S, Hiyama T, Ito M, Kitadai Y, Sumii M, et al. Hypoxia-inducible factor-1alpha expression and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncol Rep. 2003; 10:797–802. PMID:
12792726.
95. Beasley NJ, Leek R, Alam M, Turley H, Cox GJ, Gatter K, et al. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 2002; 62:2493–2497. PMID:
11980639.
96. Koukourakis MI, Giatromanolaki A, Skarlatos J, Corti L, Blandamura S, Piazza M, et al. Hypoxia inducible factor (HIF-1a and HIF-2a) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res. 2001; 61:1830–1832. PMID:
11280732.
97. Lyons JF, Wilhelm S, Hibner B, Bollag G. Discovery of a novel Raf kinase inhibitor. Endocr Relat Cancer. 2001; 8:219–225. PMID:
11566613.

98. Rapisarda A, Uranchimeg B, Scudiero DA, Selby M, Sausville EA, Shoemaker RH, et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 2002; 62:4316–4324. PMID:
12154035.
99. Geoerger B, Kerr K, Tang CB, Fung KM, Powell B, Sutton LN, et al. Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res. 2001; 61:1527–1532. PMID:
11245461.
100. Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000; 30:3–21. PMID:
10678579.
101. Koukourakis MI, Simopoulos C, Polychronidis A, Perente S, Botaitis S, Giatromanolaki A, et al. The effect of trastuzumab/docatexel combination on breast cancer angiogenesis: dichotomus effect predictable by the HIFI alpha/VEGF pretreatment status? Anticancer Res. 2003; 23:1673–1680. PMID:
12820439.
102. Fujiwara K, Kiura K, Ueoka H, Tabata M, Hamasaki S, Tanimoto M. Dramatic effect of ZD1839 ('Iressa') in a patient with advanced non-small-cell lung cancer and poor performance status. Lung Cancer. 2003; 40:73–76. PMID:
12660009.

103. Liu XH, Kirschenbaum A, Lu M, Yao S, Dosoretz A, Holland JF, Levine AC. Prostaglandin E2 induces hypoxia-inducible factor-1alpha stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem. 2002; 277:50081–50086. PMID:
12401798.
104. Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J Biol Chem. 1999; 274:32631–32637. PMID:
10551817.
105. Welsh SJ, Williams RR, Birmingham A, Newman DJ, Kirkpatrick DL, Powis G. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther. 2003; 2:235–243. PMID:
12657718.
106. Hur E, Kim HH, Choi SM, Kim JH, Yim S, Kwon HJ, et al. Reduction of hypoxia-induced transcription through the repression of hypoxia-inducible factor-1alpha/aryl hydrocarbon receptor nuclear translocator DNA binding by the 90-kDa heat-shock protein inhibitor radicicol. Mol Pharmacol. 2002; 62:975–982. PMID:
12391259.
107. Kurebayashi J, Otsuki T, Kurosumi M, Soga S, Akinaga S, Sonoo H. A radicicol derivative, KF58333, inhibits expression of hypoxia-inducible factor-1alpha and vascular endothelial growth factor, angiogenesis and growth of human breast cancer xenografts. Jpn J Cancer Res. 2001; 92:1342–1351. PMID:
11749701.
108. Kubo T, Maezawa N, Osada M, Katsumura S, Funae Y, Imaoka S. Bisphenol A, an environmental endocrine-disrupting chemical, inhibits hypoxic response via degradation of hypoxia-inducible factor 1alpha (HIF-1alpha): structural requirement of bisphenol A for degradation of HIF-1alpha. Biochem Biophys Res Commun. 2004; 318:1006–1011. PMID:
15147973.
109. Lin S, Tsai SC, Lee CC, Wang BW, Liou JY, Shyu KG. Berberine inhibits HIF-1alpha expression via enhanced proteolysis. Mol Pharmacol. 2004; 66:612–619. PMID:
15322253.
110. Welsh S, Williams R, Kirkpatrick L, Paine-Murrieta G, Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2004; 3:233–244. PMID:
15026543.
111. Chang H, Shyu KG, Lee CC, Tsai SC, Wang BW, Hsien Lee Y, et al. GL331 inhibits HIF-1alpha expression in a lung cancer model. Biochem Biophys Res Commun. 2003; 302:95–100. PMID:
12593853.
112. Iyer NV, Leung SW, Semenza GL. The human hypoxia-inducible factor 1alpha gene: HIF1A structure and evolutionary conservation. Genomics. 1998; 52:159–165. PMID:
9782081.
113. Moon EJ, Jeong CH, Jeong JW, Kim DR, Yu DY, Murakanim S, et al. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1α. FASEB J. 2004; 18:382–384. PMID:
14688211.

114. Bae MK, Jeong JW, Kim SH, Kim SY, Kang HJ, Trentin GA, et al. Tid-1 interacts with pVHL and modulates angiogenesis by destabilization of HIF-1α. Cancer res. (accepted).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download