Abstract
Purpose
In the present study, ribbon antisense to the hTR RNA, a component of the telomerase complex, was employed to inhibit telomerase activity and cancer cell growth.
Materials and Methods
Ribbon antisense molecules to the human hTR gene (hTR-RiAS) were constructed and complexed with a short modified peptide and cationic liposomes to improve the cellular uptake of the antisense molecules. The DPL complexes containing hTR-RiAS were transfected into target cancer cells. Various assays were performed to confirm the effects of the hTR-RiAS on the gene expression and cell proliferation.
Results
When cancer cells were treated with hTR-RiAS, the cellular level of hTR mRNA was reduced by more than 95%, as shown by RT-PCR. Further, the telomerase acti vity was also affected by the antisense treatment. In contrast, both mismatched and scrambled oligonucleotides failed to reduce the levels of hTR mRNA and telomerase activity. When checked for cancer cell viability, hTR-RiAS inhibited cell growth by more than 70%, in a very rapid manner. The reduced cell viability was found to be due to apoptosis of cancer cells.
Telomeres are specialized DNA structures composed of 6 bases repeat sequence (TTAGGG) at the end of each eukaryotic chromosome. This structure protects chromosomes from end to end fusion, degradation and rearrangements, thus contributing to chromosomal stabilization, which maintains the integrity of chromosomes (1). Telomeres become progressively shortened with each cell division, by 50~200 bp, due to the inability of DNA polymerase to replicate the end of the chromosomes in somatic cells. When the end of a shortened chromosome reaches a critical point, the shortening is believed to induce senescence of cells. Cellular senescence is caused by the induction of DNA damage responses, including activation of p53 or p21 (2,3).
Telomerase is a ribonucleoprotein DNA polymerase that elongates telomeric repeats of telomere in eukaryotic cells, and plays an important role in multiple cellular processes, including cell differentiation, proliferation, inhibition of apoptosis and tumorigenesis, and possibly DNA repair and drug resistance (4~7). Immortalized cells show activation of telomerase, thus maintaining the telomere structure of a chromosome (8). Telomerase activity is detected in the majority of malignant tumors, but is not detectable in normal human somatic cells. The selective expression of telomerase in tumor cells makes telomerase an attractive therapeutic target.
Human telomerase is composed of three major subunits: hTR (human telomerase RNA template) (9), hTERT (human telomerase reverse transcriptase) (10,11) and TP1 (telomerase associated protein 1) (12,13). The RNA template, hTR, contains the sequence AAUCCCAAU, through which telomerase can extend telomeric repeats. Among the 3 major components of human telomerase, antisense-based strategies have been attempted against hTR and hTERT, in both in vitro and in vivo studies, to inhibit telomerase activity (14~17). Antisense to hTR eliminates the RNA template for telomere synthesis. Tumor cells transfected with antisense hTR lose telomeric repeats, resulting in cellular senescence (18~19).
A series of antisense molecules have been developed with enhanced stability and low toxicity (20,21). Among these, ribbon antisense is the latest, which has been shown to exhibit good antisense activity, with exceptional stability, natural nucleotide composition and easy construction. Successful antisense activity is dependent on the efficient cellular uptake of antisense molecules as well as improved antisense properties. The DNA transfection mediated by cationic liposomes can be further enhanced upon forming tripartite DPL complexes that contain a short peptide of the protein transduction domain (manuscript in preparation).
In the present study, the antisense activity of hTR-RiAS was tested in several cancer cell lines that show telomerase activation. The cellular uptake of hTR-RiAS was significantly enhanced for optimal antisense activity by forming the DPL complex in vitro.
The ribbon antisense to hTR was constructed using the method described earlier (21). Oligonucleotides containing target sequences were synthesized using an Expedite 8909 DNA synthesizer (Applied Biosystem, Foster city, CA). The antisense and control (scrambled and mismatched) oligodeoxynucleotides (ODN) were phosphorylated at the 5' end. Both antisense to hTR and control ODN were used to form stemloop structures. The intra-molecular stem was formed with complementary sequences at both the 5' and 3' ends of each ODN. Two identical ODN molecules were joined by single-stranded sequences that were complementary to each other at the 5' ends. ODN molecules were mixed and heated for 2 min at 95℃, followed by gradual cooling to room temperature. One unit of T4 DNA ligase was added and incubated for 24 h at 16℃ to generate a ribbon type molecule ligated covalently with diad-symmetry. The ribbon type ODN consists of two loops connected by one stem. The ODNs were purified with a Poros HQ anion exchange column (PerSeptive Biosystems, Framingham, MA). Anion exchange was performed by gradient elution with 1M NaCl solutions. Purified ODNs were dried in an evaporator and desalted by reverse phase chromatography. Finally, the desalted ODNs were precipitated by ethanol and resuspended in ddH2O.
The cellular uptake of hTR-RiAS into cancer cells was enhanced by employing a tripartite DPL (DNA: peptide: liposomes) transfection system, which had previously been found to be effective in our laboratory. Antisense oligos were complexed with a short modified peptide of the protein transduction domain (PTD) derived from the Tat protein, and the complex was then added to cationic liposomes, Lipofectamine (Invitrogen, Carlsbad, CA), to form the DPL complex. Each component of the tripartite complex, DNA/peptide/liposomes, was mixed in the ratio of 1:3:5 (w:w:w) and added to the target cells to improve their cellular uptake of the antisense molecules.
The telomerase activity was determined using a Telo TAGGG Telomerase PCR ELISAPLUS Kit (Roche Diagnostics, Germany), as recommended by the manufacturer. HeLa cells were seeded at 1×106 cells/well in 6-well plates. The cells were transfected with hTR-RiAS and incubated for 48 h. To compare the effects of the antisense molecules on the telomerase activity, equal amounts of mismatched control or liposome alone were also added to the cells, and simultaneously assayed. The cells were then trypsinized and lysed in 500µl of ice-cold lysis buffer for 30 min. The lysates were centrifuged at 13,000 rpm and 4℃ for 30 min, the supernatants rapidly frozen and stored at -70℃. One µl of each extract, corresponding to 1×103 cells, were assayed in a total reaction mixture in 50µl to detect the telomerase activity. Telomeric repeats were added to a biotin-labeled primer during the first telomerase-mediated extension reaction. The elongation products were amplified by PTC-100 thermal cycler (MJ Research). In this step, a TS8(+ ve) template set and heat-treated HeLa cells were used as positive and negative controls for detection of telomerase expression, respectively, and simultaneously amplified. An aliquot of the PCR product was denatured, hybridized to a DIG-labeled, telomeric repeat-specific probe and bound to a streptavidin-coated 96-well plate. Finally, the immobilized PCR product was detected with an anti-DIG-POD antibody, visualized by a color reaction, using the substrate TMB, and quantified photometrically at 450 nm.
The MTT assay was performed to study the effect of hTR-RiAS molecules on the proliferation of cancer cells. HeLa cells were plated at 5×103 cells/well in a 96-well plate one day prior to transfection and incubated in an atmosphere of 5% CO2 at 37℃. The next day, the cells were washed twice with TOM-Transfection Optimized Medium (JBI, Deagu, Korea) and transfected with hTR-RiAS in a 50 µl volume for 6 h. The cells were then added with 100 µl complete medium, containing 20% FBS, and incubated for a further 4 days. The media were replaced with 50 µl of fresh medium, with the addition of 20 µl MTT reagent (5 mg/ml) prepared in phosphate buffered saline, followed by incubation in a CO2 incubator at 37℃ for 4 h. 150 µl of dimethyl sulfoxide was added to the cells and incubated for 1 h at room temperature with gentle mixing. The absorbance was measured at 570 nm with a SpectraMAX 190 (Molecular Devices, Sunnyvale, CA, USA) to quantify the surviving cells. The percentage of cell growth inhibition in each well treated with the antisense was calculated by comparing the optical density with that of a sham-treated control, using the following formula: 1 - (absorbance of an experimental well/absorbance of a control well)×100.
HeLa cells were seeded at 1×106 cells in a 6 cm-dish one day prior to hTR-RiAS treatment. To compare the effects of the antisense molecules on apoptosis, control ODNs (scrambled or mismatched) or an equal amount of liposomes alone were also added to the cells and simultaneously assayed. Cells were lysed with cell lysis buffer (1% NP-40, 25 mM EDTA, 50 mM Tris-HCl, pH 6.6) 24 h post-transfection. The cell lysates were treated with RNase A (final conc. 20 µg/ml) and incubated for 3 h at 58℃. Proteinase K was then added, to a final concentration of 20 µg/ml, and incubated for 3 h at 37℃. The cell lysates were mixed with 2.5 vol. of ice-cold ethanol and 0.1 vol. of 3M ammonium acetate and stored overnight at -20℃. The precipitated DNA was resuspended in 25 µlof TE buffer (pH 8.0) and run on a 1.8% agarose gel.
It is considered critical that antisense oligonucleotides (AS-oligos) exhibit stability toward nucleases, while maintaining sequence specific antisense activity. Ribbon antisense (RiAS) molecules, with excellent stability and antisense activity, without side effects, which have been reported with chemically modified counterparts (21).
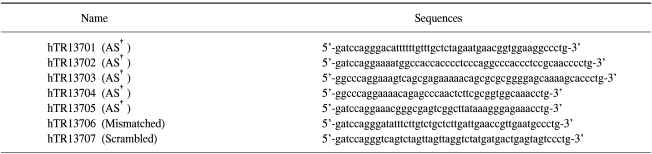
The selection of target sites may be performed by the exhaustive simulation of secondary structures of target RNA. The entire sequence of hTR RNA was examined in order to find target sites readily accessible to antisense molecules. Five target sites were chosen; named hTR13701 (50 bp), hTR13702 (52 bp), hTR13703 (52 bp), hTR13704 (43 bp) and hTR13705 (43 bp). Mismatched and scrambled control oligos were also designed and designated hTR13706 and hTR13707, respectively (50 bp). The hTR13706 oligo had 8 mismatched bases in the sequence of hTR13701 (Table 1). Antisense molecules to the five potential target sites were ligated to form RiAS molecules, having a stem and two identical antisense loops at both ends of the stem (Fig. 1).
When examined for hTR expression, all six different cancer cell lines tested were found to be positive for hTR expression (Fig. 2A). These were two lung cancer cell lines (A549 and NCIH1299), a liver cancer cell line (Hep3B), a colon cancer cell line (SW480), a cervical cancer cell line (HeLa) and a skin cancer cell line (A375SM). The five different RiAS molecules were examined for antisense activity in HeLa cells. Twenty-four hours post-transfection, the total RNA was purified and subjected to RT-PCR. Among the 5 molecules, AS13701 was able to eliminate hTR mRNA to completion (Fig. 2B). The ribbon antisense was termed hTR-RiAS, and adopted for further studies.
To achieve successful antisense activity, it is critical to have efficient uptake of antisense oligos. The tripartite DPL (DNA/peptide/liposomes) complex found effective for the cellular uptake of both plasmid DNA and AS oligos (manuscript in preparation), was employed. The DPL complex was formed with hTR-RiAS, for enhanced transfection, and tested in various cancer cell lines in an effort to reduce the cellular level of hTR RNA. When hTR-RiAS was used for antisense activity in HeLa cells, the antisense was found to be especially potent, almost completely ablating cellular hTR RNA (Fig. 3A). hTR-RiAS was able to reduce hTR RNA by about 60% at a concentration of 0.1 µg and by 80% at 0.5 µg. Almost complete ablation of hTR RNA was observed with 1.0 µg of hTR-RiAS. The RT-PCR product obtained was then examined for sequence authenticity by Southern blotting. The DNA band was probed with a 27 mer internal primer (5'-CTCGCTGACTTTCAGCGGGC GGAAAAG-3') labeled with FITC. The data confirmed that the amplified DNA band was derived from hTR RNA (data not shown). A similar level of antisense activity was also observed in another two cancer cell lines (SW840 and NCIH1299) tested, indicating a broad utility of hTR-RiAS to various cancer cells (Fig. 3B and 3C). In contrast, both scrambled and mismatched oligos exhibited only mild reductions of hTR RNA. Treatment of liposomes alone also showed no reduction in the level of hTR RNA. Transfection of hTR-RiAS did not affect theβ-actin expression. The effect on the telomerase activity in HeLa cells treated with the hTR-RiAS was also examined. The cells treated with hTR-RiAS showed more than a 60% reduction in their telomerase activity compared with the cells treated with liposomes alone, mismatched oligos, or with the sham treated cells (Fig. 4).
These results confirm that the hTR-RiAS has specific and potent antisense activity to hTR RNA when efficiently delivered as a DPL complex.
The potency of the antisense activity of hTR-RiAS was then tested for inhibition of HeLa cell growth. The cells treated with increasing amounts (0.05 µg, 0.1 µg and 0.2 µg) of hTR-RiAS showed growth inhibition of about 35, 50 and 70%, respectively, in proportion to the increasing amounts of the antisense transfection (Fig. 5). In contrast, control treatments showed no significant growth inhibition.
It has been reported that treatment of antisense to telomerase complexes, including hTR RNA, can cause apoptosis (5). The cell death caused by hTR-RiAS treatment was investigated to see if it reflected the induction of apoptosis. For this, HeLa cells treated with the antisense molecules were subjected to a DNA fragmentation assay. The hTR-RiAS was found to cause characteristic DNA ladder formation 24 h after transfection, indicating apoptotic progression (Fig. 6). No DNA ladder formation was detected in the control treated cells when using scrambled oligos, mismatched oligos or liposomes alone.
In the present study, the potent antisense activity of ribbon antisense to hTR has been demonstrated when the cellular uptake of the antisense was improved by the tripartite DPL system. Since hTR is an essential component of the human telomerase complex, targeting hTR for the inhibition of telomerase activity has been attempted with different gene silencing: antisense, ribozymes, and more recently siRNA approaches (22~24). Recent investigations have indicated that the inhibition of telomerase activity might change the growth mechanism of cancer cells and suppress tumor growth (25).
Telomerase activity is detected in 85~90% of human tumors, but not in most somatic cells, indicating a broad involvement of hTR expression in human malignancies. The hTR-RiAS in six human cancer cell lines were tested for telomerase expression. The enhanced uptake of hTR-RiAS resulted in almost complete ablation of hTR RNA, effective inhibition of telomerase activity and blockade of cancer cell proliferation. Thus, an effective approach for eliminating a component of the telomerase complex may prove efficacious for a broad spectrum of human cancers.
The enhanced properties of ribbon antisense ODN have been reported. RiAS ODN has a covalently closed structure, with much enhanced stability as well as natural nucleotide composition. Conventional AS oligos with enhanced stability have adopted chemical modifications that have been blamed for various side effects. It has been reported that conventional antisense oligos require larger amounts of antisense oligos, ranging from 20 to 200 µg/ml, to cause biological effects that may not entirely be sequence specific. Various control and real time PCR data shown in this report demonstrate that hTR RiAS was effective, specifically in a lesser amount. The improved antisense activity shown in this report can be explained, not only by the improved properties of AS-oligos, but also in the enhanced cellular uptake.
In general, antisense oligos show poor cellular uptake due to anionic charges on their polymeric backbone. To improve the cellular uptake of antisense oligos, the DPL transfection system has been adopted, and has shown 70~90% cell positivity for AS-oligos uptake, resulting in significant improvement of transfection efficiency in vitro. However, the transfection system needs further improvement to be effective for systemic applications.
Targeting telomerase in an effective manner would have potential utility in the treatment of various human cancers, as telomerase is ubiquitously expressed in human tumors. hTR-RiAS was shown to be effective in ablating telomerase activity and in the suppression of tumor growth. It would be interesting to search for a way to totally blockade tumor growth by further improvement of the cellular uptake of the AS-oligos or in some combination with conventional therapeutic modalities.
Ribbon antisense molecules to the human hTR gene were complexed with a short modified peptide and cationic liposomes, which was employed to inhibit telomerase activity and cancer cell growth. When cancer cells were treated with hTR-RiAS, the cellular levels of hTR RNA and telomerase activity were affected by the antisense treatment. When the cancer cell viability was checked, hTR-RiAS was found to inhibit cell growth by more than 70% in a very rapid manner. The reduced cell viability was found to be due to apoptosis of cancer cells. These results show that hTR-RiAS is a powerful anticancer reagent, with the potential for broad efficacy to diverse malignant tumors.
References
2. Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990; 345:458–460. PMID: 2342578.

3. Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai Ev, Futcher AB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 1992; 89:10114–10118. PMID: 1438199.

4. Urquidi V, Tarin D, Goodison S. Role of telomerase in cell senescence and oncogenesis. Annu Rev Med. 2000; 51:65–79. PMID: 10774453.

5. Fu W, Begley JG, Killen MW, Mattson MP. Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem. 1999; 274:7264–7271. PMID: 10066788.

6. Nugent CI, Bosco G, Ross LO, Evans SK, Salinger AP, Moore JK, Haber JE, Lundblad V. Telomere maintenance is dependent on activities required for end repair of double strand breaks. Curr Biol. 1998; 8:657–660. PMID: 9635193.
7. Ishikawa T, Kamiyama M, Hisatomi H, Ichikawa Y, Momiyama N, Hamaguchi Y, Hasegawa S, Narita T, Shimada H. Telomerase enzyme activity and RNA expression in adriamycin-resistant human breast carcinoma MCF-7 cells. Cancer Lett. 1999; 141:187–194. PMID: 10454261.

8. Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994; 266:2011–2015. PMID: 7605428.

9. Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, et al. The RNA component of human telomerase. Science. 1995; 269:1236–1241. PMID: 7544491.

10. Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997; 90:785–795. PMID: 9288757.

11. Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997; 277:955–959. PMID: 9252327.

12. Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science. 1997; 275:973–977. PMID: 9020079.

13. Nakayama J, Saito M, Nakamura H, Matsuura A, Ishikawa F. TLP1: a gene encoding a protein component of mammalian telomerase is a novel member of WD repeats family. Cell. 1997; 88:875–884. PMID: 9118230.

14. Mukai S, Kondo Y, Koga S, Komata T, Barna BP, Kondo S. 2-5A antisense telomerase RNA therapy for intracranial malignant gliomas. Cancer Res. 2000; 60:4461–4467. PMID: 10969793.
15. Herbert B, Pitts AE, Baker SI, Hamilton SE, Wright WE, Shay JW, Corey DR. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA. 1999; 96:14276–14281. PMID: 10588696.

16. Glukhov AI, Zimnik OV, Gordeev SA, Severin SE. Inhibition of telomerase activity of melanoma cells in vitro by antisense oligonucleotides. Biochem Biophys Res Commun. 1998; 248:368–371. PMID: 9675142.
17. Norton JC, Piatyszek MA, Wright WE, Shay JW, Corey DR. Inhibition of human telomerase activity by peptide nucleic acids. Nat Biotechnol. 1996; 14:615–619. PMID: 9630953.

18. Naka K, Yokozaki H, Yasui W, Tahara H, Tahara E, Tahara E. Effect of antisense human telomerase RNA transfection on the growth of human gastric cancer cell lines. Biochem Biophys Res Commun. 1999; 255:753–758. PMID: 10049783.

19. Kondo S, Tanaka Y, Kondo Y, Hitomi M, Barnett GH, Ishizaka Y, Liu J, Haqqi T, Nishiyama A, Villeponteau B, Cowell JK, Barna BP. Antisense telomerase treatment: induction of two distinct pathways, apoptosis and differentiation. FASEB J. 1998; 12:801–811. PMID: 9657520.

20. Moon IJ, Lee Y, Kwak CS, Lee JH, Choi K, Schreiber AD, Park JG. Target site search and effective inhibition of leukaemic cell growth by a covalently closed multiple anti-sense oligo-nucleotide to c-myb. Biochem J. 2000; 346:295–303. PMID: 10677346.
21. Moon IJ, Choi K, Choi YK, Kim JE, Lee Y, Schreiber AD, Park JG. Potent growth inhibition of leukemic cells by novel ribbon-type antisense oligonucleotides to c-myb1. J Biol Chem. 2000; 275:4647–4653. PMID: 10671493.

22. Kondo S, Kondo Y, Li G, Silverman RH, Cowell JK. Targeted therapy of human malignant glioma in mouse model by 2-5A antisense directed against telomerase RNA. Oncogene. 1998; 16:3323–3330. PMID: 9681832.
23. Kanazawa Y, Ohkawa K, Ueda K, Mita E, Takehara T, Sasaki Y, Kasahara A, Hayashi N. Hammerhead ribozyme-mediated inhibition of telomerase activity in extracts of human hepa tocellular carcinoma cells. Biochem Biophys Res Commun. 1996; 225:570–576. PMID: 8753802.
24. Kosciolek BA, Kalantidis K, Tabler M, Rowley PT. Inhibition of telomerase activity in human cancer cells by RNA interference. Mol Cancer Ther. 2003; 2:209–216. PMID: 12657713.
25. Kondo Y, Koga S, Komata T, Kondo S. Treatment of prostate cancer in vitro and in vivo with 2-5A-anti-telomerase RNA component. Oncogene. 2000; 19:2205–2211. PMID: 10822370.
Fig. 1
Schematic diagram for the construction of ribbon antisense to the hTR gene. hTR13701 was phosphorylated at the 5' end, and harbors complementary sequences at both 5' and 3' ends, with a single stranded sequence of GATC at the 5' extreme end. Ribbon antisense was constructed by covalently ligating two identical hTR13701 molecules. The RiAS oligos, therefore, contains two loops and a stem.
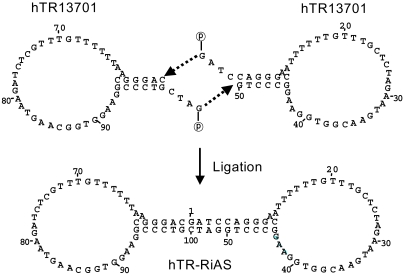
Fig. 2
hTR expression and specific antisense activity of ribbon antisense to hTR RNA. (A) Expression of hTR in various cancer cell lines. Expression of hTR was examined in 6 cancer cell lines by RT-PCR. Lane M, DNA size marker of 100 bp ladder; lane 1, A549; lane 2, NCI H1299; lane 3, Hep3B; lane 4, SW480; lane 5, HeLa and lane 6, A375SM. (B) Specific reduction of hTR RNA in HeLa cells by ribbon antisense to hTR RNA. Cells were transfected with a tripartite DPL complex (hTR-RiAS/Tatpeptide/Lipofectamine). Five different RiAS oligos were used, and RT-PCR assays performed. Lane M, 100 bp ladder; lane 1, sham; lane 2, liposomes alone; lane 3, hTR13701; lane 4, hTR13702; lane 5, hTR13703; lane 6, hTR13704 and lane 7, hTR13705.
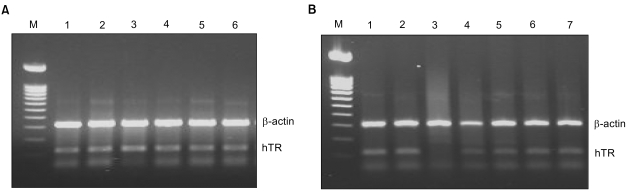
Fig. 3
Specific reduction of hTR RNA by hTR-RiAS in various cancer cell lines. After transfecting a tripartite DPL complex (hTR-RiAS/Tat-peptide/Lipofectamine) into each target cell, RT-PCR was performed. (A) Dose dependent specific reduction of hTR RNA by hTR-RiAS in HeLa cells. Lane M, 100 bp DNA ladder; lane 1, sham; lane 2, liposome alone; lane 3, hTR-RiAS (0.1 µg); lane 4, hTR-RiAS (0.5 µg) and lane 5, hTR-RiAS (1.0 µg). (B) Specific reduction of hTR RNA by hTR-RiAS in SW480 cells: Lane M, 100 bp DNA ladder; lane 1, sham; lane 2, liposome alone; lane 3, scrambled control (1.0 µg); lane 4, mismatched control (1.0 µg) and lane 5, hTR-RiAS (1.0 µg). (C) Specific reduction of hTR RNA by hTR-RiAS in NCIH1299 cells. Lane M, 100 bp DNA ladder; lane 1, sham; lane 2, liposome alone; lane 3, scrambled control (1.0 µg); lane 4, mismatched control (1.0 µg) and lane 5, hTR-RiAS (1.0 µg).

Fig. 4
Reduction of telomerase activity in hTR RiAS-treated HeLa cells. After transfecting a tripartite DPL complex (hTR-RiAS/Tat-peptide/Lipofectamine) into HeLa cells, the telomerase activity was detected using the TRAP-ELISA method. Cells that were sham-treated, treated with liposomes alone, with mismatched control. A TS8 template set and heat-treated HeLa cells were used as positive and negative controls for the detection of telomerase expression, respectively. Each bar value represents the mean±S.D. of triplicate experiments. *Abbreviation: Lipo., liposomes alone
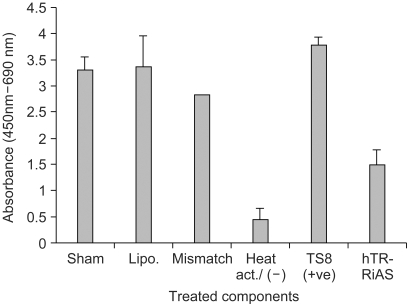
Fig. 5
Effect of hTR-RiAS on the proliferation of HeLa cells. The cells were treated with tripartite DPL complexes containing 0.05, 0.1 and 0.2 µg of hTR-RiAS, as indicated. The transfectants were examined for growth inhibition by an MTT assay 72 h post-transfection. Cells were sham-treated and treated with liposomes alone and simultaneously assayed. Each bar value represents the mean±S.D. of triplicate experiments. *Abbreviation: Lipo., liposomes alone

Fig. 6
Induction of apoptotic DNA ladder formation by hTR RiAS in HeLa cells. Genomic DNA was extracted in 48 h treatment of tripartite DPL complexes and run on a 1.8% agarose gel. Lane M, 100 bp DNA ladder; lane 1, sham; lane 2, liposomes alone; lane 3, scrambled control; lane 4, mismatched control and lane 5, hTR-RiAS.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download