Abstract
Purpose
We conducted a phase II study of docetaxel and ifosfamide chemotherapy for patients with platinum-resistant or refractory non-small-cell lung cancer (NSCLC) to evaluate the response and toxicity profiles as a salvage treatment.
Materials and Methods
Between July 2000 and July 2004, 40 patients who had previously received platinum-based regimen as the first-line or second-line therapy were enrolled in this study. The treatment consisted of a docetaxel 75 mg/m2 intravenous infusion on day 1 and intravenous ifosfamide 3 g/m2 with Mesna® uroprotectione on day 1 through 3. This regimen was repeated every 3 weeks.
Results
One hundred thirty cycles of treatment were given, with a median of 3 cycles (range: 2~6 cycles). All the patients were evaluable for the response rate and toxicity profile. The major toxicity was myelosuppression. Grade 3~4 neutropenia occurred in 30 patients (75%) during treatment. Febrile neutropenia occurred in 16 patients (40%). Five of 40 patients (12.5%) had a partial response (95% confidence interval, 3.3~21.7%). The median time to disease progression was 2.65 months (range: 2.02~3.20 months), and the median survival was 5.24 months (range: 2.99~7.49 months).
Lung cancer is the leading cause of cancer-related death in South Korea and throughout the world, and the incidence of this disease is continuing to increase (1). The majority of these cases are diagnosed when the disease is at a locally advanced or metastatic stage (2). In addition, more than 50% of patients who undergo surgery have recurring, unresectable tumors or they experience extrathoracic dissemination. As a consequence, this means that systemic chemotherapy plays an important role in more than three-quarters of all the patients having NSCLC at sometime during the course of their disease (3). Currently, first-line platinum-based chemotherapy is usually recommended for those patients with a good performance status as the routine care of advanced NSCLC. However, virtually all patients who respond initially to this therapy will eventually become refractory.
The value of chemotherapy in a salvage setting for the patient who has failed with primary chemotherapy is debatable. However, docetaxel has been found to have activity for salvage chemotherapy of NSCLC in several phase II studies, and it could prolong patient survival, as has been shown in phase III randomized trials that have compared docetaxel versus vinorelbine or ifosfamide, or docetaxel versus the best supportive care, for NSCLC patients who have failed with previous chemotherapy (4~6).
Ifosfamide is an alkylating drug that has demonstrated activity against NSCLC (7,8). Used as a single agent in patients with advanced NSCLC, ifosfamide has demonstrated activity, with a response rate of 20 to 25% and a median survival time of 9 months (7,8); thus, it is considered as one of the most active drugs against NSCLC (7~9).
Because the majority of patients with advanced NSCLC are given standard platinum-based chemotherapy, it appears particularly attractive to combine docetaxel and ifosfamide as a non-platinum-based regimen.
In this current Phase II study, we evaluated the activity and safety of a docetaxel and ifosfamide salvage regimen for patients with refractory or resistant NSCLC that progressed during or after the standard first-line or second-line platinum-based chemotherapy.
The patients for this study were enrolled between July 2000 and July 2004 at Gyeong-Sang National University Hospital. The eligibility criteria included patients who had been previously treated with platinum-based combination chemotherapy; they had histologically or cytologically proven unresectable or metastatic NSCLC, their ages were <75 years, they had an ECOG performance status (PS)≤2, bidimensionally measurable disease, adequate renal and liver function (normal serum creatinine and creatinine clearance>60 ml/min, total bilirubin<2 mg/dl, serum AST and ALT<3 times the upper limit of normal), and adequate bone marrow function (neutrophils≥1,500/ul, platelet count≥100,000/ul, hemoglobin≥10 g/dl). Those patients with peripheral neuropathy, symptomatic brain metastases, active infection, active coronary artery disease, unstable diabetes mellitus and active concomitant malignancy were excluded from the study. An informed consent was obtained from all the patients and this study was approved by the institutional review board at Geyong-Sang National University Hospital.
Based on the Dutch phase I study (10), the selected eligible patients were treated as follows: docetaxel was administered at 75 mg/m2 over a 1 hour period by intravenous infusion on day 1. Ifosfamide was administered at 3,000 mg/m2 intravenously over a 1 hour on day 1 through 3 together with Mesna® uroprotection, 40% of the ifosfamide dose, given intravenously before, at 3 and 6 hours after ifosfamide. The treatment was preceded by the parenteral administration of antiemetics consisting of 5-HT3 receptor antagonists plus dexamethasone (20 mg intravenously), and this was followed by 4 days of orally administered antiemetics for the prevention of delayed emesis. The cycles were repeated every 3 weeks.
Blood cell counts, serum creatinine and liver function tests were performed before each course of chemotherapy. In the case of grade III or IV hematologic toxicity, the doses of ifosfamide and docetaxel were reduced by 20% for the subsequent courses. For renal toxicity of grade 3 or higher (serum creatinine elevations>3 x normal), the treatment was withheld until the test results normalized, and a 20% dose reduction of ifosfamide was applied in the subsequent chemotherapy courses. For neuropathy grade III or higher, the treatment was interrupted. For grade III or higher mucositis, the doses of docetaxel and ifosfamide were reduced by 20% in the subsequent cycles.
In the case that the blood counts had not recovered to an absolute neutrophil count (ANC)≥1,500/Ul and a platelet count ≥100,000/ul on the day of therapy, the treatment was withheld until recovery. Clinical toxicities were assessed according to the modified National Cancer Institute Common Toxicity Criteria.
Those patients who received at least 2 cycles of treatment were evaluable for response according to the WHO criteria unless clinical evidence of tumor progression occurred after the first cycle (11), and the patients who received at least 1 cycle of treatment were evaluable for drug toxicity.
The sample size chosen for the study was calculated from an expected response rate of 25% and a minimum response rate of 10% with an α=0.05 and β=0.2, using Simon's two-stage minimax design (12).
At the first stage of the trial, if fewer than 2 responses occured out of the first 22 patients, then the study would conclude that the anticipated response rate (RR) is <10%, and the study would be terminated.
The response duration was measured from the day of its initial documentation until there was confirmed disease progression, and time to progression (TTP) was calculated from the date of the first cycle of chemotherapy until there was documented evidence of progressive disease (PD). Overall survival was measured from the starting date of salvage chemotherapy until the last follow-up or until the patient's death. Survival rates and TTP were assessed by the Kaplan-Meier product-limit method.
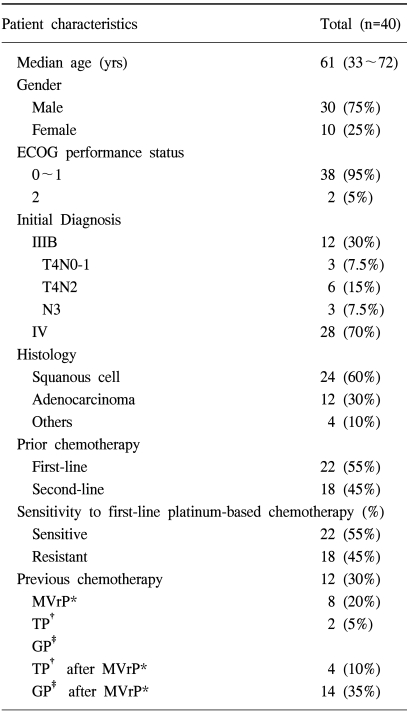
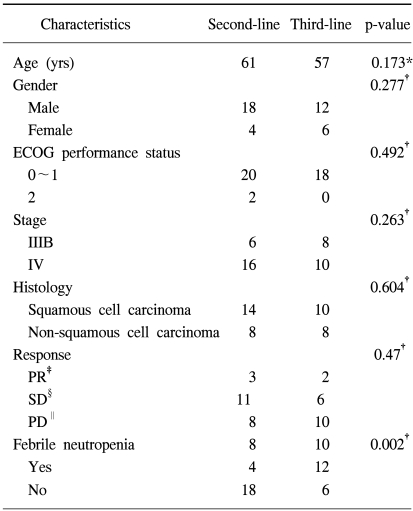
Forty patients with previously treated advanced NSCLC were entered into the current study, and their characteristics are shown in Table 1. The median age of the patients was 61 years (range; 33~72 years). Thirty patients were male and ten patients were female. There were 12 patients with stage IIIB disease and 28 patients with stage IV disease at the time of the initial diagnosis. Forty patients received at least two cycles of therapy, and so they were evaluable for their response and for drug toxicity.
The objective responses included partial response (PR) in 5 of 40 patients (12.5%; 95% confidence interval [95% CI], 3.3~21.7%), stable disease (SD) in 17 of 40 patients (42%; 95% confidence interval [95%CI], 28.3~55.7%), and progressive disease (PD) in 18 of 40 patients (45%; 95% confidence interval [95%CI], 31.2~58.8%). The response in patients who were resistant to first-line chemotherapy occurred for 2 of 16 patients (12.5%), whereas the response in patients with sensitive disease was 3 of 24 patients (12.5%), and these results did not differ significantly (p=0.120). The median TTP was 2.06 months (range: 2.02~3.20 months) (Fig. 1). The median survival was 5.24 months (range: 2.99~7.49 months) (Fig. 2).
In this study, the docetaxel and ifosfamide salvage regimen was given to 22 patients as a second-line chemotherapy and to 18 patients as a third-line chemotherapy. There were no significant differences for the patients' baseline characteristics and response rates between the two groups (Table 2).
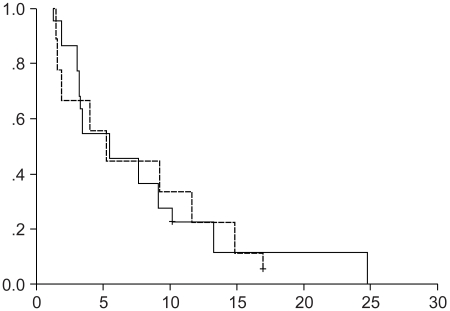
The median survival time was 5.44 and 5.24 months for the second-line group (range, 2.09~8.79 months), and the third-line group (range, 2.65~7.83 months), respectively (Fig. 3, Log-rank test, p=0.964). However, there was a higher proportion of febrile neutropenia observed in the third-line chemotherapy group (67%, 12 of 18), as compared with the second-line chemotherapy group (18%, 4 of 22) (p=0.002).
130 courses of drug therapy were delivered. The actual administered median dose-intensities were 17.5 mg/m2/week for docetaxel (range: 15.5~21.0 mg/m2/week; relative dose intensity (RDI), 70%) and 710 mg/m2/week for ifosfamide (range: 690~790 mg/m2/week; RDI 71%).
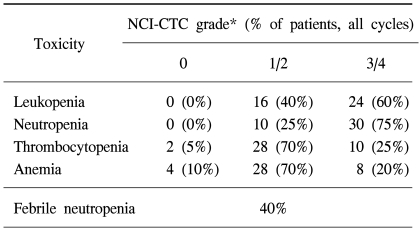
All of the 40 patients enrolled into the study were eligible for toxicity evaluation. The main toxicity observed was a hematolgic condition. Grade 3~4 neutropenia was seen in 30 of 40 patients (75%), with 16 o1f 40 patients (40%) developing grade 4 neutropenia. All the patients with grade 4 neutropenia developed neutropenic fever, and this was successfully managed with broad-spectrum antibiotics what were administered with granulocyte-colony stimulating factor (G-CSF). Grade 3~4 anemia and thrombocytopenia were observed in 20% and 25% of the patients, respectively (Table 3).
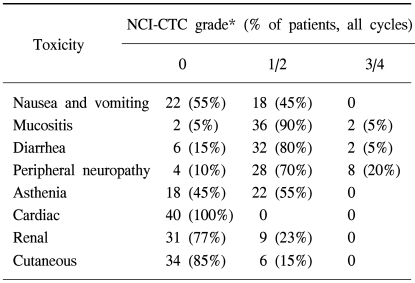
Grade 3 peripheral neuropathy and grade 3 mucositis were observed in 8 patients (20%) and 2 patients (5%), respectively, whereas any other grade 3~4 non-hematologic toxicities were not encountered. Mild asthenia (grade 1~2) was seen in 55% of the patients as the major non-hematologic toxicities (Table 4).
Subsequent treatments in all patients were interrupted by grade 3 peripheral neuropathies. However, grade 3~4 hematologic toxicities and other grade 3 non-hematologic toxicities were not a limiting factor for interrupting the subsequent cycles.
An increasing proportion of patients with advanced NSCLC will derive clinical benefit and prolonged survival from frontline treatment with several novel drugs such as vinorelbine, paclitaxel, gemcitabine and platinum combinations, and it is anticipated that many of these patients will require salvage chemotherapy after they display platinum-based chemo-resistant or chemo-refractory disease (12).
Docetaxel appears to represent a new drug with a single-agent activity rate of 17% for cisplatin-pretreated patients with NSCLC, and docetaxel has an RR approaching that of the most active drugs used in first-line treatment (13). This was confirmed in an extended, multicenter, phase II trial involving 80 patients with platinum-refractory or platinum-resistant NSCLC where an RR of 16% was obtained, and there was no impact of the platinum sensitivity status on the RR (14). Docetaxel has the advantage of a longer intracellular half-life that leads to higher intracellular concentrations than paclitaxel (15), and it is 100 times more potent than paclitaxel with respect to bcl-2 phosphorylation (16).
For this reason, docetaxel has promising activity for patients with untreated (17,18) and platinum-resistant NSCLC (5,13,14). In the preclinical studies, ifosfamide showed an interesting activity against the NSCLC human xenograft (19).
In the phase III studies of chemotherapy for NSCLC, the best results have been obtained with regimens that contained ifosfamide (7,20). Moreover, there is a lack of cross-resistance between docetaxel and the alkylating agents such as ifosfamide, which have shown synergistic antitumor activity in vivo (21,22).
For docetaxel in combination with other agents, a phase I study with docetaxel and ifosfamide for patients with advanced solid tumors have suggested that a 75 mg/m2 dose of docetaxel combined with 5.0 g/m2 dose of ifosfamide appeared to be manageable (10).
Based on these results, several trials were performed to evaluate the activity and toxicity of a docetaxel and ifosfamide combination for NSCLC and other malignancy. A phase II study of docetaxel and ifosfamide as a second-line treatment for 22 patients with advanced or metastatic urothelial cancer, after their failure with platinum chemotherapy, was found to be effective with an acceptable toxicity profile (23).
In another study using docetaxel plus ifosfamide and cisplatin in chemo-naïve NSCLC patients, it was shown that the majority of the chemotherapy courses had to be supported by G-CSF administration, and the clinical efficacy of this regimen does not seem to justify the toxicity profile (24).
Taiwanese phase II study on docetaxel and ifosfamide combination chemotherapy for NSCLC patients who failed previous chemotherapy with or without paclitaxel as a salvage setting revealed a relatively low response rate, a low dose intensity, and high proportion of severe neutropenia in NSCLC patients (25).
Our study also demonstrated a low response rate (12.5%) and a high rate of hematologic toxicities compared with docetaxel monotherapy. This low response rate may have been caused by the higher proportion of the third-line treatment group (45% of patients), the interruption of subsequent cycles due to grade 3 peripheral neuropathy (20% of patients), and the low dose intensities of docetaxel and ifosfamide.
According to Japanese studies (24), Taiwanese studies (25) and our study, far-east Asian patients appear to have poorer tolerance and greater hematologic toxicities with docetaxel-based treatment compared with western patients. Taiwanese and Japanese studies have also concluded that the addition of ifosfamide to docetaxel for NSCLC patients should not be recommended either as the first-line or second-line chemotherapy due to the severe hematologic toxicities (24,25). Based on our study, the hematologic toxicity for the docetaxel and ifosfamide combination chemotherapy was just too severe to continue applying this treatment in this salvage setting. Further investigation will be needed to find reasonable dosages for docetaxel and ifosfamide, and to discover other feasible drugs that can be combined with docetaxel or ifosfamide as a better salvage regimen.
Our study showed that this salvage regimen displayed a relatively low antitumor activity compared with only docetaxel treatment, and there was a high proportion of severe neutropenia in the previously treated advanced NSCLC patients. Physicians should also be aware of dosage adjustment, the selection of patients and the risk of treatment related morbidity in salvage chemotherapy with docetaxel and ifosfamide for patients with NSCLC.
References
1. Bae JM, Won YJ, Jung KW, Suh KA, Yun YH, Shin MH, Ahn YO, Lee DH, Shin HR, Ahn DH, Oh DK, Park JG. Survival of Korean cancer patients diagnosed in 1995; 134 Korean Cancer Registry-affiliated Hospitals. Cancer Res Treat. 2002; 34:319–325.
2. Vokes EE, Bitran JD, Vogelzang NJ. Chemotherapy for non-small-cell lung cancer: The continuing challenge. Chest. 1991; 99:1326–1328. PMID: 1645242.
3. Grilli R, Oxman AD, Julian JA. Chemotherapy for advanced non-small cell lung cancer: How much benfit is enough? J Clin Oncol. 1993; 11:1866–1872. PMID: 8410111.
4. Fossella FV, Rigas J. The use of docetaxel (Taxotere) in patients with advanced non-small cell lung cancer previously treated with platinum-containing chemotherapy regimens. Semin Oncol. 1999; 26:9–12. PMID: 10458204.
5. Fossella FV, DeVore R, Kerr RN, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, Gandara D, Karp D, Vokes E, Kris M, Kim Y, Gamza F, Hammershaimb L. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000; 18:2354–2362. PMID: 10856094.
6. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J. Prosepctive randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000; 18:2095–2103. PMID: 10811675.
7. Sculier JP, Paesmans M, Thiriaux J, Lecomite J, Bureau G, Giner V, Efremidis A, Lafitte JJ, Berchier MC, Alexopoulos CG, Zacharias C, Mommen P, Ninane V, Klastersky J. Phase III randomized trial comparing cisplatin and carboplatin with or without ifosfamide in patients with advanced non-small-cell lung cancer. European Lung Cancer Working Party. J Clin Oncol. 1998; 16:1388–1396. PMID: 9552042.

8. Johnson DH. Overview of ifosfamide in small cell and non-small cell lung cancer. Semin Oncol. 1990; 6:87–98.
9. Paccagnella A, Favaretto A, Brandes A, Ghiotto C, Fornasiero A, Volpi A, Pappagallo G, Festi G, Cipriani A, Vinante O. Cisplatin, etoposide, and ifosfamide in non-small cell lung carcinoma. A phse II randomized study with cisplatin and etoposide as the control arm. Cancer. 1990; 65:2631–2634. PMID: 2160312.
10. Pronk LC, Schrikvers D, Schellens JH, de Brujin EA, Planting AS, Locci-Tonelli D, Groult V, Verweij J, van Oosterom AT. Phase I study on docetaxel and ifosfamide in patients with advanced solid tumors. Br J Cancer. 1998; 77:153–158. PMID: 9459161.

11. World Health Organization. The WHO Handbook for reporting the results of Cancer Treatment. 1979. Geneva: World Health Organization.
12. Simon R. How large should a phase II trial of a new drug be? Cancer Treat Rep. 1987; 71:1079–1085. PMID: 3315196.
13. Kosmas C, Tsavaris N, Mylonakis N, Kalofonos HP. An overview of current results with the gemcitabine and docetaxel combination as initial and salvage chemotherapy regimen in advanced non-small cell lung cancer. Crit Rev Oncol Hematol. 2003; 45:265–275. PMID: 12633839.

14. Fossella FV, Lee JS, Shin DM, Calayag M, Huber M, Perez-Soler R, Murphy WK, Lippman S, Benner S, Glisson B. Phase II study of docetaxel for advanced or metastatic platinum-refractory non-small-cell lung cancer. J Clin Oncol. 1995; 13:645–651. PMID: 7884425.

15. Gandara DR, Vokes E, Green M, Bonomi P, Devore R, Comis R, Carbone D, Karp D, Belani C. Activity of docetaxel in platinum-treated non-small-cell lung cancer: results of a phase II multicenter trial. J Clin Oncol. 2000; 18:131–135. PMID: 10623703.
16. Diaz JF, Andreu JM. Assembly of purified GDP-tubulin into microtubule induced by taxol and taxotere: reversibility, ligand stoichiometry, and completition. Biochemistry. 1993; 32:2747–2755. PMID: 8096151.
17. Haldar S, Basu A, Croce C. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997; 57:229–233. PMID: 9000560.
18. Cerny T, Kaplan S, Pavlidis N, Schoffski P, Epelbaum R, van Meerbeek J, Wanders J, Franklin HR, Kaye S. Docetaxel (Taxotere) is an active in non-small cell lung cancer: a Phase II trial of the EORTC Early Clinical Trials Group (ECTG). Br J Cancer. 1994; 70:384–387. PMID: 7914429.
19. Francis PA, Rigas JR, Kris MG, Pisters KM, Orazem JP, Woolley KJ, Heelan RT. Phase II Trial of docetaxel in patients with stage III and IV non-small-cell lung cancer. J Clin Oncol. 1994; 12:1232–1237. PMID: 7911159.

20. Berger DP, Fiebig H, Winterhalter BR, Wallbrecher E, Henss H. Preclinical phase II study of ifosfamide in human tumor xenografts in vivo. Cancer chemother Pharmacol. 1990; 26:S7–S11. PMID: 2347054.
21. Drings P, Buchholz E, Manegold C. Ifosfamide and docetaxel in non-small cell lung cancer. Semin Oncol. 1998; 25(1 Suppl 2):29–37. PMID: 9535209.
22. Sommer K, Peters SO, Robins IH. A preclinical model for experiemental chemotherapy of human head and neck cancer. Int J Oncol. 2001; 18:1145–1149. PMID: 11351243.
23. Krege S, Rembrink V, Borgermann C, Otto T, Rubben H. Docetaxel and ifosfamide as second-line treatment for patients with advanced or metastatic urothelial cancer after failure of platinum chemotherapy: a phase 2 study. J Urol. 2001; 165:67–71. PMID: 11125366.
24. Kunitoh H, Akiyama Y, Kusaba H, Yamamoto N, Sekine I, Ohe Y, Kubota K, Tamura T, Shinkai T, Kodama T, Goto K, Niho S, Nishiwaki Y, Saiko N. A phase I/II trial of cisplatin, docetaxel and ifosfamide in advanced or recurrent non-small cell lung cancer. Lung Cancer. 2001; 33:259–265. PMID: 11551421.

25. Chen YM, Shih JF, Lee CS, Chen MC, Lin WC, Tsai CM, Perng RP. Phase II study of docetaxel and ifosfamide combination chemotherapy in non-small-cell lung cancer patients failing previous chemotherapy with or without paclitaxel. Lung Cancer. 2003; 39:209–214. PMID: 12581575.

Fig. 1
Kaplan-Meier curve for the time to progression. The median time to progression was 2.06 months (range: 2.02~3.20 months).
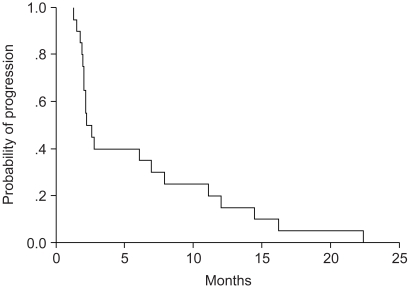
Fig. 2
Kaplan-Meier curve for overall survival. The median duration of overall survival was 5.24 months (range: 2.99~7.49 months).
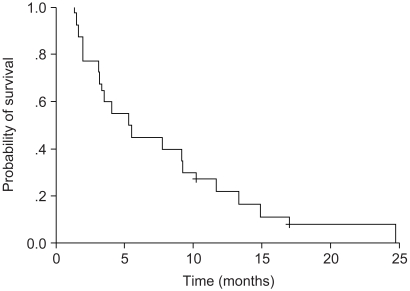
Fig. 3
The Kaplan-Meier survival curve for the second-line group versus the third-line group. Solid line: The second-line group (the median survival duration was 5.44 months, the range was 2.09~8.79 months); Broken line: The third-line group (the median survival duration was 5.25 months, the range was 2.65~7.83 months) (Log-rank test, p=0.964).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download