INTRODUCTION
MATERIALS AND METHODS
1) Preparation of a unidirectional subtracted liver cDNA library
2) Construction of a liver-specific random gene LC-antisense library
3) Transfection of the LC-antisense library into HepG2 cells
4) Cell proliferation assay
5) Gene identification and sequence motif search
6) Cell cycle analysis
7) Apoptotic DNA fragmentation analysis
RESULTS
1) Construction of an LC-antisense library
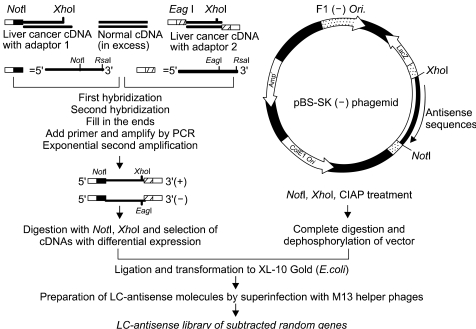 | Fig. 1A schematic diagram for the construction of a random gene LC-antisense library. Total RNA was prepared from a pair of liver normal and cancer tissues, with both mRNA populations converted into cDNA. The tester cDNA (from cancer tissue) and driver cDNA (from normal tissue) were digested with RsaI to obtain shorter and blunt-ended fragments. Two hybridization reactions were performed, followed by suppression PCR to selectively amplify sequences overexpressed or expressed only in liver cancer cells, but not in normal liver cells. The subtracted cDNA pool was cloned into the multicloning site of the pBS SK (-) vector in the same direction as the LacZ gene. The cDNA plasmid library was transformed into E.coli competent cells. LC-antisense molecules were prepared on a large scale by superinfecting competent bacterial cells with a helper phage, and arrayed in 96-well plates. |
2) Identification of genes involved in liver cancer cell growth
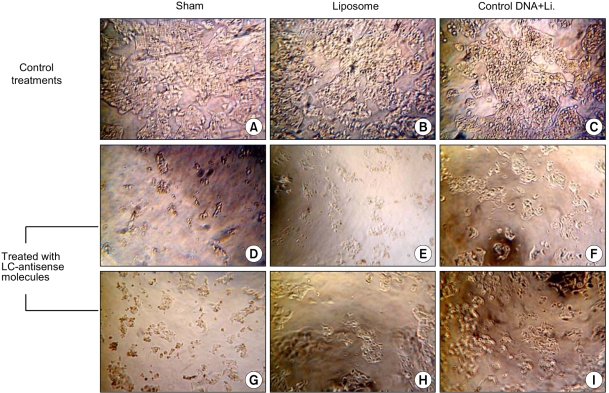 | Fig. 2Microscopic observations after LC-antisense transfection to HepG2 cells. Growth Inhibitions of HepG2 cells were examined by light microscopy 4 days post transfection of the LC-antisense library (×200). (A)-(C); control treatments as indicated, (D)-(I); HepG2 cells treated with six different LC-antisense molecules. In this figure, the data acquired from the treatments with 6 out of 1,200 LC-antisense types are representatively shown as an example. *Li., liposome |
3) Effects of LC-antisense on cell cycle progression and apoptotic induction
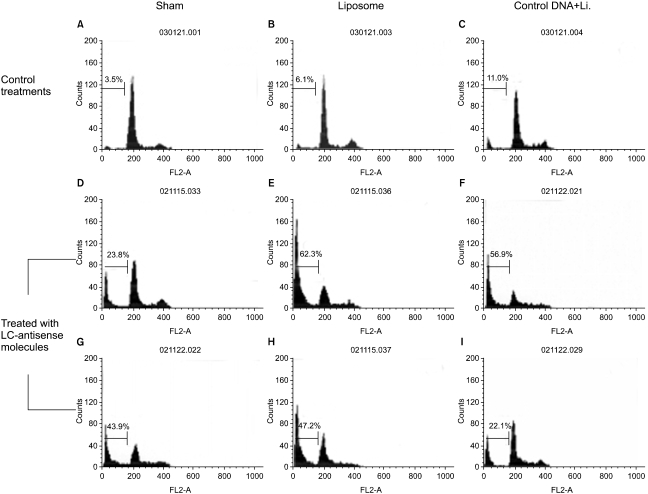 | Fig. 4Effects of LC-antisense molecules on the cell cycle progression. The LC-antisense molecules of 58 genes were transfected into HepG2 cells along with the controls. Cells were harvested 48 h post transfection. Functional analysis was perfomed on an equal number of cells (104 events) by flow cytometry after DNA staining with propidium iodide. In this figure, the data acquired from the treatments with 6 out of 58 LC-antisense types are representatively shown as an example. (A)-(C) control treatments as indicated; (D) LCAS 11; (E) LCAS 14; (F)LCAS 15; (G) LCAS 16; (H) LCAS 29; (I) LCAS 31. *Li., liposome |
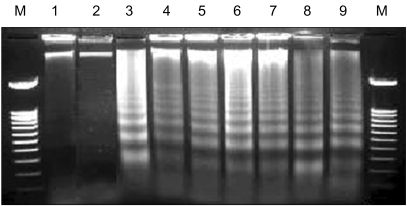 | Fig. 5Induction of apoptotic DNA ladder formation by LC-antisense molecules. The LC-antisense molecules of 58 genes were transfected into HepG2 cells along with the controls. Genomic DNA was extracted 48 h post transfection and run on a 1.6% agarose gel. In this figure, the data acquired from treatments with 6 out of 58 LC-antisense types are representatively shown as an example. Lane M, 100 bp DNA ladder size marker; lane 1, sham treatment; lane 2, control DNA+liposome complexes; lane 3, LCAS 11; lane 4, LCAS 14; lane 5, LCAS 15; lane 6, LCAS 16; lane 7, LCAS 21; lane 8, LCAS 29; lane 9, cisplatin (positive control). |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download