Abstract
Purpose
This study was performed to evaluate the treatment results, prognostic factors and complication rates in patients with locally advanced cancer of uterine cervix after radiotherapy with high-dose rate (HDR) brachytherapy.
Materials and Methods
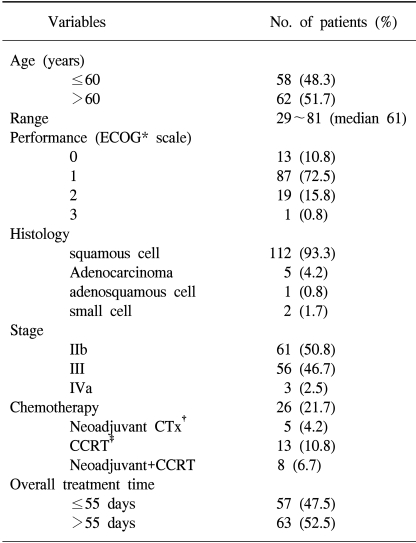
One hundred and twenty patients with a locally advanced (stages IIB~IVA according to FIGO classification) carcinoma of the uterine cervix were treated with radiotherapy at the Department of Radiation Oncology, Samsung Medical Center between September 1994 and December 2001. The median age of the patients was 61 years (range 29 to 81). Sixty-one, 56 and 3 patients had FIGO stage IIB, III, and IV diseases, respectively. All patients were given external beam radiotherapy over the whole pelvis (median 50.4 Gy) and HDR intracavitary brachytherapy, with a median of 4 Gy per fraction, to point A. Twenty-one patients received chemotherapy, of which 13 and 21 received neoadjuvant chemotherapy and concurrent chemotherapy, respectively, during the first and fourth weeks of external beam radiotherapy. The chemotherapy was not randomly assigned and the median follow-up time was 28.5 months (range: 6~100 months).
Results
The three- and 5-year overall survival (OS) and disease-free survival (DFS) rates were 64.4 and 57.0%, and 63.7 and 60.2%, respectively. The 5-year OS and DFS rates of the patients at stages IIB, III and IV were 60.2, 57.9 and 33.3%, and 57.4, 65.4 and 33.3%, respectively. Univariate analysis indicated that the FIGO stage, overall treatment time (OTT) and treatment response were significant variables for the OS (p=0.035, p=0.0649 and p=0.0009) and of the DFS (p=0.0009, p=0.0359 and p=0.0363). Multivariate analysis showed that the treatment response was the only significant variable for the OS (p=0.0018) and OTT for the DFS (p=0.0360). The overall incidence of late complications in the rectum and bladder were 11.7 and 6.7%, respectively. In addition, insufficiency fractures were observed in 7 patients (5.8%).
The incidence of carcinoma of the uterine cervix in Korea is decreasing, but still the third most common malignant neoplasm in women (1). Radiotherapy is the treatment of choice in patients with an advanced cancer of the uterine cervix, and normally consists of a combination of external beam radiotherapy (EBRT) and intracavitary brachytherapy. However, the optimal combination of these methods has not been clearly defined. According to the Patterns of Care studies in the United States, the recurrences and complications are reduced when brachytherapy is used in combination with EBRT (2). HDR brachytherapy was developed to overcome the potential disadvantages of low-dose rate (LDR) brachytherapy (radiation exposure to medical staff, prolonged treatment time, mandatory hospitalization and applicator movement). In 2000, the American Brachytherapy Society recommended certain guidelines for HDR brachytherapy of a carcinoma of the cervix (3). HDR brachytherapy has been used successfully in Japan and Europe for the last 30 years, and has been widely used in Korea since 1979 (4).
The results of the use of radiotherapy for the patients with a cancer of the uterine cervix have previously been reported (5). The aims of this study were to analyze the long-term disease control, survival and prognostic factors in patients with advanced cancer of the uterine cervix treated by EBRT and HDR intracavitary brachytherapy.
Between September 1994 and December 2001, 269 patients were diagnosed with cancer of the uterine cervix at the Samsung Medical Center. Of these, 120 patients, who were in stages IIB~IVA, according to the FIGO classification, were treated with radical radiotherapy. All patients had no history of other malignancy and had an intact uterus. The median age of the patients was 61 years, ranging from 22 to 81 years, with sixty-two (51.7%) older than 60 years. The ECOG performances were 0 in 13 patients (10.8%), 1 in 87 (72.5%), 2 in 19 (15.8%) and 3 in one (0.8%). The histologies were squamous and non-squamous cell carcinomas in 112 (93.3%) and 8 (6.7%) patients, respectively. The pre-treatment evaluations included the patients' medical history, a physical examination, blood tests, punch biopsy, chest X-ray, bimanual examination, IVP, rectosigmoidoscopy, cystoscopy and either a pelvic MRI or CT scan. The patients were staged according to the guidelines from the Federation of Gynecology and Obstetrics (FIGO). Sixty-one, 56 and 3 patients had FIGO stages, III and IV diseases, respectively (Table 1).
All patients were given EBRT to the whole pelvis as well as HDR intracavitary brachytherapy. Each patient received EBRT to the pelvis and the paraaortic lymph nodes if gross paraaortic lymph nodes were observed in the MRI or CT scan. Eleven patients received paraaortic lymph nodes irradiation. The whole pelvis total dose ranged from 45.0 to 55.8 Gy, with a median dose of 50.4 Gy. The total dose to the paraaortic nodes was 45 Gy. The Daily fraction was 1.8 Gy, administered 5-times a week. In order to reduce the overall treatment time (OTT), 6 patients not receiving chemotherapy took EBRT 6-times-a-week. The patients were irradiated with a 4-field box technique (anterior (AP), posterior (PA) and bi-laterals) in order to spare some of the small bowel anterior to the iliac nodes. However, the anterior and posterior opposed fields were used when the paraaortic nodes were included. Each patient received individualized radiation fields according to the extent of the disease on either the CT or MRI scan. The general treatment fields are prescribed below: The superior border of the whole pelvis fields was the L5-S1 level in the negative pelvic node patients and L4-L5 in the positive pelvic nodes patients. The superior border of the paraaortic nodes was the T12-L1 level. The inferior border was placed 2~3 cm below the lowest extent of the cervical or vaginal disease. For the AP-PA fields, the lateral borders were placed approximately 1.5~2 cm lateral to the inner bony margins of the true pelvis. Usually, the anterior border included half of the posterior one-third of the symphysis pubis, and the posterior border was located at the S2-3 level. Mid-line shielding was added after 40~45 Gy. For patients with a disease extending to the parametrium or pelvic side wall(s), a parametrial boost (using the AP/PA fields with mid-line shielding), with a median dose of 8 Gy (range: 6~10 Gy) in 1.8~2 Gy fractions was given. External beam radiotherapy was performed using a 10~15 MV linear accelerator. In order to reduce acute complications of the small bowel, a small bowel displacement device was used (6).
HDR brachytherapy was started 4 to 5 weeks after the initiation of the external beam radiotherapy. One week before the HDR brachytherapy, an MRI was taken to evaluate the response to radiation and for brachytherapy planning. The equipment used for the HDR brachytherapy was the Microselectron® (Nucletron, The Netherlands), with Iridium-192. Orthogonal films were taken to verify the placement of the applicators and to perform the dosimetric plan. Packing was always performed in order to fix the applicators and to maximize the distance between the sources and the posterior rectal wall and anterior bladder. The dose was prescribed at point A, according to the International Commission on Radiation Units and Measurements recommendations (7). The median dose of HDR brachytherapy was 24 Gy (range: 12~24 Gy) at point A, with 4 Gy per fraction twice a week for 3 weeks.
The overall treatment time of radiation (OTT) ranged from 42 to 180 days (median 56 days), and 57 patients (47.5%) completed the radiotherapy within 55 days (Table 1).
Twenty-one patients (21.7%) received chemotherapy, of witch 5 received neoadjuvant chemotherapy, 13 received concurrent chemotherapy, during the 1st and 4th weeks of external beam radiotherapy, and 8 both neoadjuvant and concurrent chemotherapy (Table 1).
The patients were followed up by a radiation oncologist and a gynecologist 1 month after completing the radiotherapy, every 3 months during the first 2 years, and every 6 months thereafter. A clinical examination and cervical cytology were performed at each follow-up. An MRI was performed prior to HDR brachytherapy, and at 1 and 12 months after the completion of the radiotherapy. The median follow-up time was 28.5 months (range 6~100 months).
The overall and disease-free survivals were calculated according to the Kaplan-Meier method. A comparison of the categorical variables was performed using the chi-square test.
A gynecological examination and MRI were used to evaluate the response to radiotherapy 1 month after its completion. Eight-three patients (69.2%) achieved complete remission and 37 (30.8%) achieved partial remission (Table 2).
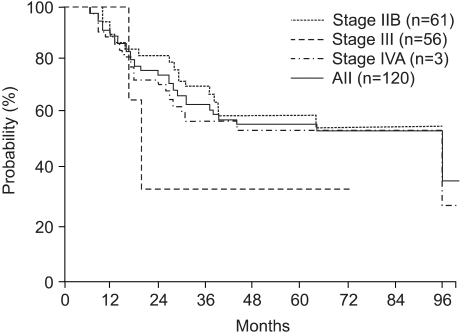
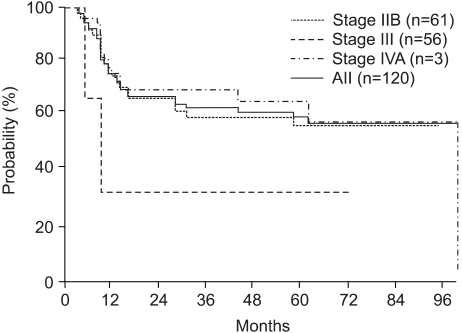
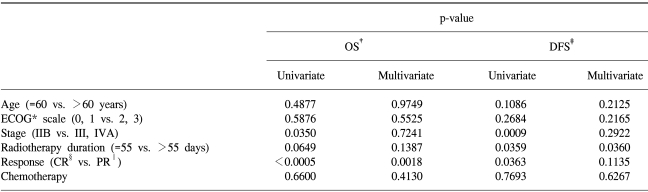
The overall survival rates at 3 and 5 years for all patients were 64.4 and 57.0%, respectively. The overall survival rates at 3 and 5 years for Stage IIB, III and IVA patients were 71.2 and 60.2%, 58.6 and 57.9% and 33.3 and 33.3%, respectively (Fig. 1). The disease-free survival rates at 3 and 5 years for all patients were 63.7 and 60.2%, respectively. The disease-free survival rates at 3 and 5 years for the Stage IIB, III and IVA patients were 60.5 and 57.4%, 70.0 and 65.4% and 33.3 and 33.3%, respectively (Fig. 2). A Cox multiple regression analysis showed that the FIGO stage, OTT and treatment response were significant variables for the overall (p=0.035, p=0.0649 and p=0.0009) and disease-free survivals (p=0.0009, p=0.0359 and p=0.0363) by a univariate analysis. A multivariate analysis showed that the treatment response was the only significant variable for the overall survival (p=0.0018), and the OTT was the only significant variable for disease-free survival (p=0.0360) (Table 3). Of eleven patients with positive paraaortic lymph nodes, 10 (18%) had stage IIB and 11 (18%) stage III diseases. The response and overall and disease-free survival rates are not significant different between the two groups.
Twenty-nine patients (24.2%) developed late complications after the radiotherapy. Rectal, urinary and pelvic bone late complications were observed in 14 (11.7%), 8 (6.7%) and 7 (5.8%) patients, respectively. According to the Franco-Italian glossary grade (8), Grades 1, 2 and 3 late rectal complications were observed in 9 (7.5%), 4 (3.3%) and 1 patient (0.8%), respectively. Radiation cystitis was observed in 6 patients (5.0%), vesicovaginal fistula in 1 (0.8%) and urinary stricture in 1 (0.8%). Pelvic insufficiency fractures were observed in 7 patients (in the sacrum with 4, the pubis in 1 and in both the sacrum and pubic bone in 2), but all improved with conservative management.
The treatment of choice for most patients with locoregionally advanced disease (IIB-IVA) is radiation therapy using a combination of EBRT and brachytherapy. The importance of brachytherapy in the curative treatment of cervical cancer is indisputable. Reports from the Patterns of Care studies in the United States have demonstrated significantly better survival rates for patients treated with a combination of EBRT and intracavitary brachytherapy (2).
For many years, the standard treatment for patients with a locally advanced carcinoma of the uterine cervix was EBRT to the pelvis and low-dose-rate (LDR) brachytherapy (9,10). However, the worldwide use of HDR brachytherapy as an alternative to LDR brachytherapy has increased rapidly in recent years. HDR brachytherapy was developed to overcome the potential disadvantages of LDR brachytherapy (radiation exposure to the medical staff, prolonged treatment time, mandatory hospitalization, and the risk of applicator movement). HDR brachytherapy has been used successfully in Japan and Europe for more than 30 years, and has also been used widely in Korea since 1979 (4). Although the HDR brachytherapy equipment and Iridium-192 source are expensive, and the source needs to be replaced every 3~4 months, there are many advantages of using HDR brachytherapy. Because the HDR treatment can be delivered with remote afterloading equipment, the radiation exposure to personnel is eliminated. In addition, HDR treatment requires shorter treatment times, the patients suffer less discomfort from prolonged bed rest, the patients do not need to be hospitalized, and the risk of applicator movement during treatment is reduced. It allows the integration of EBRT and HDR brachytherapy, which can lead to a shorter overall treatment duration and for potentially better tumor control. According to the reports by the Korean Society of Therapeutic Radiology and Oncology, HDR brachytherapy was performed on 1,258 Korean patients with uterine cervical cancer in 1997, with over 30 Korean institutions having HDR treatment equipment (4).
Several reports from Korean institutions concerning the treatment outcomes of uterine cervix cancer with HDR brachytherapy have been published (9,11,12). There results are comparable with those of other institution in Korea as well as those from radiotherapy with LDR brachytherapy (4).
The HDR fractionation schedules reported in the literature vary markedly, with insertion numbers and Point A fraction sizes ranging from 2 to 7 sessions and 3 to 14 Gy, respectively (13,14). In Korea, the patients usually received 6 to 8 fractions of HDR treatments, with fraction sizes ranging from 3 to 5 Gy (11). The fractionation schedule in this study was 6 to 8 fractions each of 4 Gy to point A. There are, to date, no reports of a standard treatment schedule in Korea. The use of HDR brachytherapy has gradually been increasing in Korea, but the optimal treatment regimen needs to be defined. These studies show that HDR brachytherapy with 4 Gy per fraction twice a week for 3 weeks to point A is an acceptable treatment with comparable complication rates.
Retrospective studies have demonstrated that a prolongation of OTT is associated with a reduction in both local control and survival (15~17). Most studies looking at this effect on a carcinoma of the cervix suggest a loss of local control of around 1% for each day added to an OTT of 49~52 days. Petereit et al. (15) reported that the 5-year survival and pelvic control rates differed significantly with treatment times <55 days vs. ≥55 days: 65 and 54% (p = 0.03), 87 and 72% (p=0.006), respectively. In addition, survival was decreased by 0.6%/day and pelvic control by 0.7%/day for each additional day of treatment beyond 55 days for all stages of the disease. Delaloye et al (16) and Lanciano et al (17) suggested that shorter treatment duration is a factor associated with longer survival and pelvic control in a carcinoma of the cervix. In order to shorten the OTT, brachytherapy could be performed at or near the end of EBRT. In this institution, brachytherapy was initiated during the 4th to 5th week of treatment in order to avoid exceeding a total treatment time of 45~50 days. Currently, most authorities consider a treatment duration of 55 days or less to be acceptable (18). The current study also reported that an OTT over 55 days decreased the disease-free survival and pelvic control rates.
In 1999, the results of five multiinstitutional randomized controlled trials demonstrated a survival advantage for concurrent chemoradiotherapy (CCRT) in the management of cervix cancer (18). Pelvic recurrences, as well as distant metastases were fewer and the recurrence-free interval longer in the cisplatin group than in the control group. All these trials demonstrate that CCRT with cisplatin increases the survival in women with a locally advanced cervical carcinoma. The findings of these trials prompted the US National Cancer Institute (NCI) to issue a rare clinical announcement urging that strong consideration be given to adding chemotherapy to radiation therapy in the treatment of invasive cervical cancer (19). The impact of this statement was that many clinicians altered their pattern of practice and questioned the value of continued participation in clinical trials containing radiotherapy alone or that did not utilize cisplatin as the chemotherapeutic agent of choice. Because of the small number of patients receiving chemotherapy, the current studies did not show a significant difference in the overall survival and local control between radiotherapy alone and CCRT. However, due to the announcement of the US NCI, now chemotherapy was combined with radiotherapy in patients with positive pelvic or paraaortic lymph nodes in the CT or MRI scan, a bulky disease or stage IIB, III and IV diseases in our institution.
However, the results of the National Cancer Institute (NCI) of Canada trial did not show a benefit in either pelvic control or survival by the addition of concurrent weekly cisplatin chemotherapy, with the author insisting it was important that careful attention be paid to the RT to achieving the optimum outcome (20). Potter and Knocke (21) pointed out that the results of the studies performed in North America (radiotherapy alone) were significantly worse than those published by some experienced groups in Europe and Japan, and recommend the following be taken into account when considering chemoradiotherapy for patients with cervix cancer: the high potential of radiotherapy alone; the high potential for the improvement of local control using 3-D conformal radiotherapy (3-D CRT); the significant potential of adverse side effects in a chemoradiotherapy regimen.
In recent years, with the advent of computer-based treatment planning and new technology in the field of radiology, 3-D CRT, intensity-modulated radiotherapy (IMRT) planning have been reported in the treatment of gynecological malignancies (22). The authors also reported several studies on the efficacy of a small-bowel displacement system in 3-D CRT and IMRT (23,24). Also, several investigators have evaluated the use of CT-based, MRI-based and PER-based treatment planning for brachytherapy procedures to improve local control and treatment-related toxicity (25).
The results of these studies suggest that external beam radiotherapy with HDR brachytherapy is an acceptable treatment for a locally advanced cancer of the uterine cervix. Also, OTT and treatment response were significant factors for the survival. So excessively protracting treatment (>8 weeks) should be avoided.
According to these studies, the treatment schedule with 50.4 Gy in 28 fractions of EBRT to the pelvis and 6 fractions (twice a week) of 4 Gy HDR brachytherapy was appropriate for the treatment of locally advanced uterine cervix cancer with reasonable complication rates. However, further efforts should be made to define the optimal treatment regimen and reduce the treatment time.
References
1. Shin HR, Won YJ, Jung KW, Park JG. 2001 Annual Report of the Korea Central Cancer Registry: Based on Registered Data from 134 Hospitals. Cancer Res Treat. 2004; 36:19–30.

2. Coia L, Won M, Lanciano R, Marcial VA, Martz K, Hanks G. The patterns of care outcome study for cancer of the uterine cervix. Results of the second national practice survey. Cancer. 1990; 66:2451–2456. PMID: 2249184.

3. Nag S, Erickson B, Thomadsen B, Orton C, Demanes JD, Petereit D. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000; 48:201–211. PMID: 10924990.

4. Huh SJ. Current status of high dose rate brachytherapy in cervical cancer in Korea and optimal treatment schedule. J Korean Soc Ther Radiol Oncol. 1998; 16:357–366.
5. Huh SJ, Kim BK, Lim DH, Shin SS, Lee JE, Kang MK, Ahn YC. Treatment results of radical radiotherapy in uterine cervix cancer. J Korean Soc Ther Radiol Oncol. 2002; 20:237–245.
6. Huh SJ, Lim DH, Ahn YC, Kim DY, Kim MK, Wu HG, Choi DR. Effect of customized small bowel displacement system in pelvic irradiation. Int J Radiat Oncol Biol Phys. 1998; 40:623–727. PMID: 9486612.

7. International Commission on Radiation Units and Measurements. Report 38, Dose and Volume Specifications for Reporting Intracavitary Therapy in Gynecology. 1985. Bethesda MD: International Commission in Radiation Units and Measurements.
8. Chassagne D, Sismondi P, Horiot JC, Sinistrero G, Bey P, Zola P, Pernot M, Gerbaulet A, Kunkler I, Michel G. A glossary for reporting complications of treatment in gynecological cancers. Radiother Oncol. 1993; 26:195–202. PMID: 8316648.

9. Kim OB, Choi TJ, Kim JH, Lee HJ, Kim YA, Suh YW, Lee TS, Cha SD. Carcinoma of uterine cervix treated with high dose rate intracavitary irradiation: pattern of failure. J Korean Soc Ther Radiol Oncol. 1993; 11:369–376.
10. Perez CA, Breaux S, Madoc-Jones H, Bedwinek JM, Camel HM, Purdy JA, Walz BJ. Radiation therapy alone in the treatment of carcinoma of uterine cervix. I. Analysis of tumor recurrence. Cancer. 1983; 51:1393–1402. PMID: 6402291.

11. Lee SW, Suh CO, Chung EJ, Kim GE. Dose optimization of fractionated external radiation and high-dose-rate intracavitary brachytherapy. Int J Radiat Oncol Biol Phys. 2002; 52:1338–1344. PMID: 11955747.
12. Huh SJ. The result of curative radiotherapy for carcinoma of uterine cervix. J Korean Soc Ther Radiol Oncol. 1993; 11:143–149.
13. Orton CG, Seyedasdr M, Somnay A. Comparison of high and low dose rate remote afterloading for cervix cancer and the importance of fractionation. Int J Radiat Oncol Biol Phys. 1991; 21:1425–1434. PMID: 1938550.

14. Eifel PJ. High dose rate brachytherapy for carcinoma of the cervix: High tech or high risk? Int J Radiat Oncol Biol Phys. 1996; 24:383. PMID: 1526879.
15. Petereit DG, Sarkaria JN, Chappell R, Fowler JF, Hartmann TJ, Kinsella TJ, Stitt JA, Thomadsen BR, Buchler DA. The adverse effect of treatment prolongation in cervical carcinoma. Int J Radiat Oncol Biol Phys. 1995; 32:1301–1307. PMID: 7635769.

16. Delaloye JF, Coucke PA, Pampallona S, Peltecu G, De Grandi P. Radiation therapy duration influences overall survival in patients with cervical carcinoma. Int J Gynaecol Obstet. 1997; 57:295–303. PMID: 9215493.

17. Lanciano RM, Won M, Coia LR, Hanks GE. Pretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix: a final report of the 1973 and 1978 patterns of care studies. Int J Radiat Oncol Biol Phys. 1991; 20:667–676. PMID: 2004942.

18. Lehman M, Thomas G. Is concurrent chemotherapy and radiotherapy the new standard of care for locally advanced cervical cancer? Int J Gynecol Cancer. 2001; 11:87–99. PMID: 11328406.

19. National Cancer Institute. Concurrent chemoradiation for cervical cancer. 1999. 2. 22. Washington D.C:
20. Pearcey R, Brundage M, Drouin P, Jeffrey J, Johnston D, Lukka H, MacLean G, Souhami L, Stuart G, Tu D. Phase III Trial Comparing Radical Radiotherapy With and Without Cisplatin Chemotherapy in Patients With Advanced Squamous Cell Cancer of the Cervix. J Clin Oncol. 2002; 20:966–972. PMID: 11844818.

21. Potter R, Knocke TH. The potential of 3-D conformal brachytherapy and external beam radiotherapy in cervical cancer. Strahlenther Onkol. 2001; 177:641–642. PMID: 11789402.
22. Heron DE, Gerszten K, Selvaraj RN, King GC, Sonnik D, Gallion H, Comerci J, Edwards RP, Wu A, Andrade RS, Kalnicki S. Conventional 3D conformal versus intensity-modulated radiotherapy for the adjuvant treatment of gynecologic malignancies: a comparative dosimetric study of dose-volume histograms small star, filled. Gynecol Oncol. 2003; 91:39–45. PMID: 14529660.
23. Kang MK, Huh SJ, Han Y, Park W, Ju SG, Kim KJ, Lee JE, Park YJ, Nam HR, Kim DH, Ahn YC. Small bowel sparing effect of small bowel displacement system in 3D-CRT and IMRT for cervix cancer. J Korean Soc Ther Radiol Oncol. 2004; 22:130–147.
24. Huh SJ, Park W, Ju SG, Lee JE, Han Y. Small-bowel displacement system for the sparing of small bowel in three-dimensional conformal radiotherapy for cervical cancer. Clin Oncol. 2004; (article in press).

25. Malyapa RS, Mutic S, Low DA, Zoberi I, Bosch WR, Laforest R, Miller TR, Grigsby PW. Physiologic FDG-PET three-dimensional brachytherapy treatment planning for cervical cancer. Int J Radiat Oncol Biol Phys. 2002; 54:1140–1146. PMID: 12419441.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download